Abstract
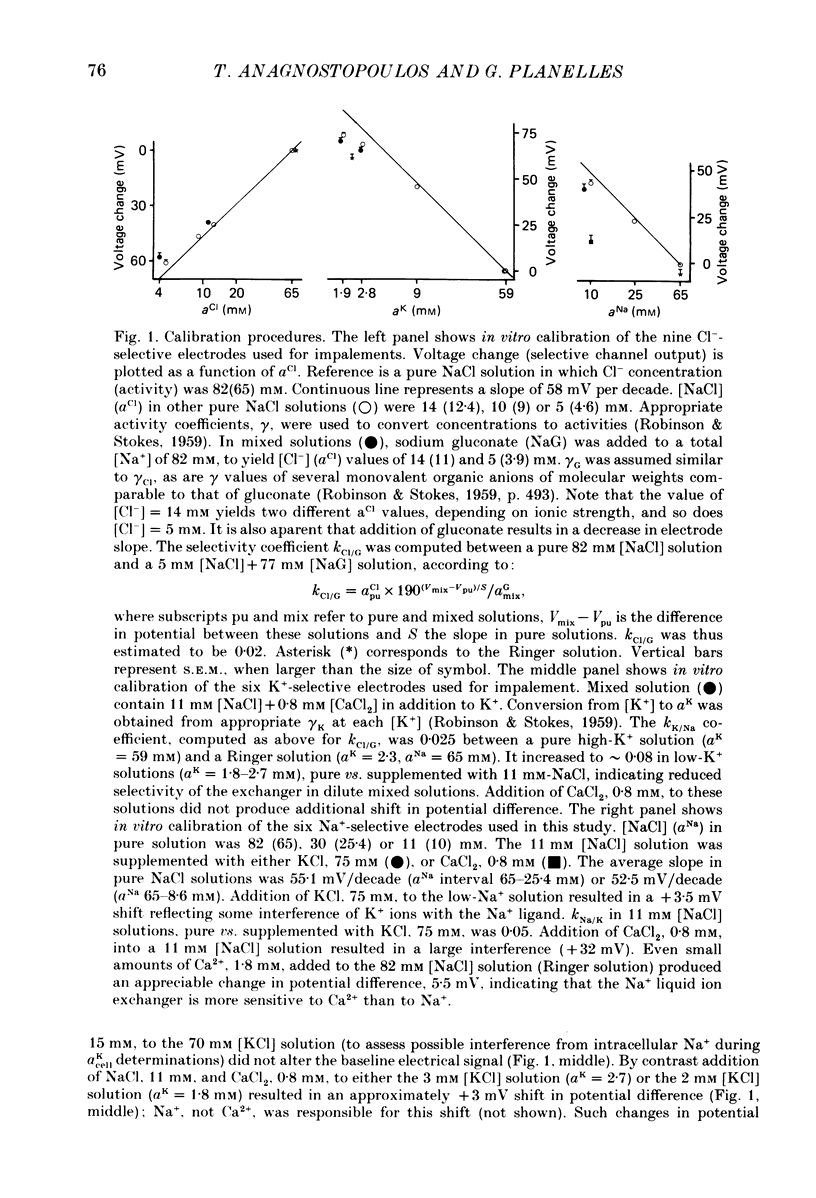
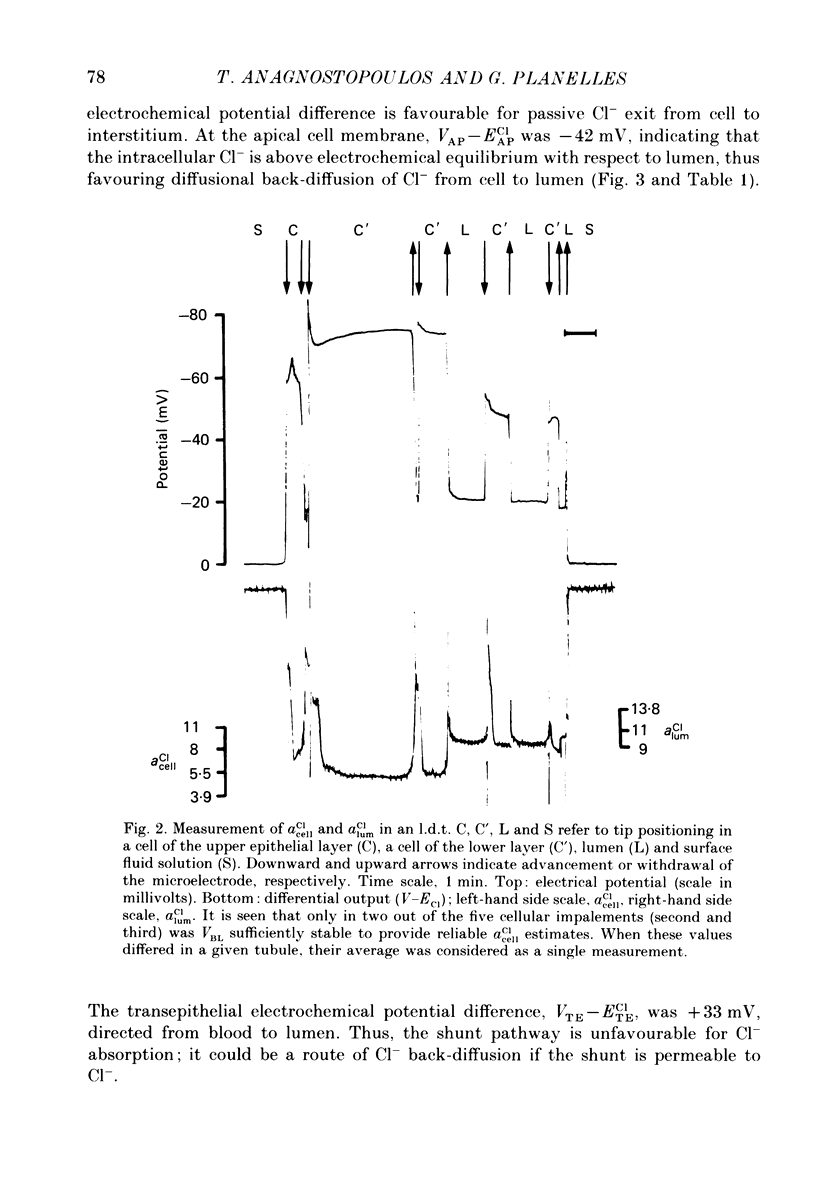
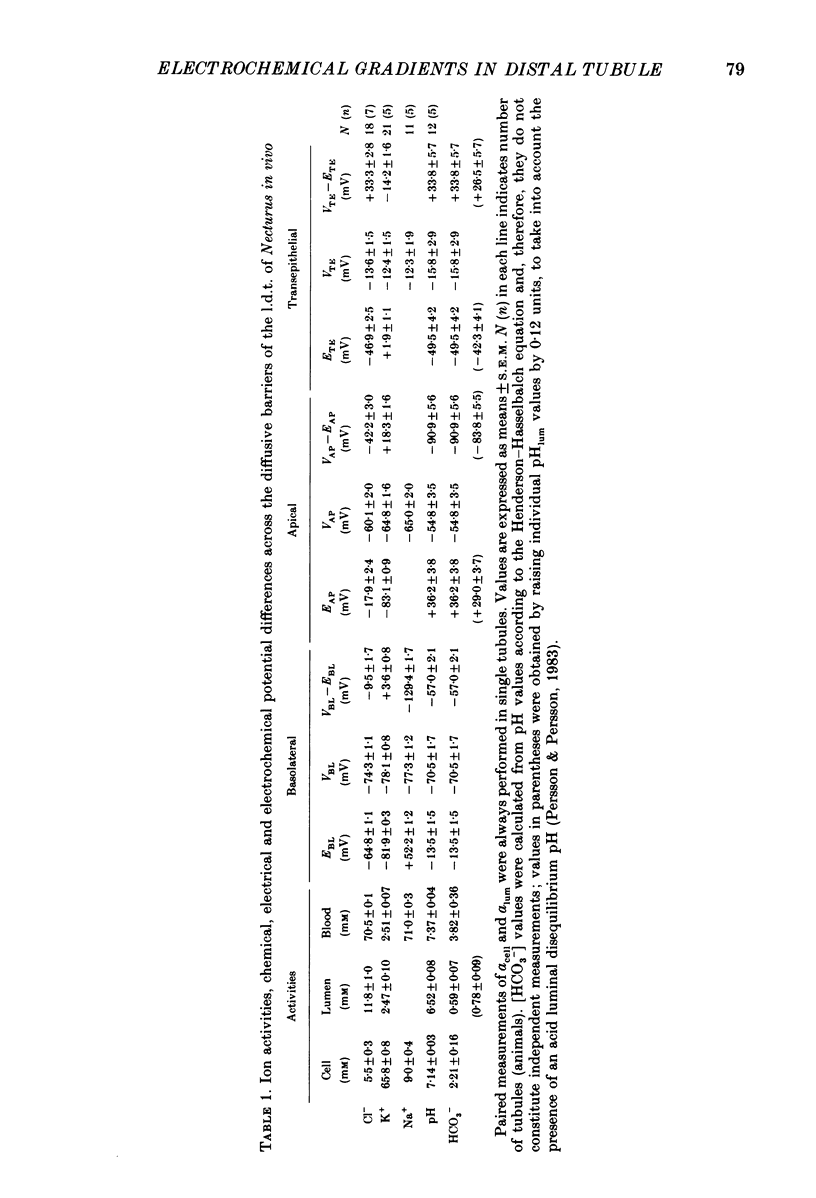
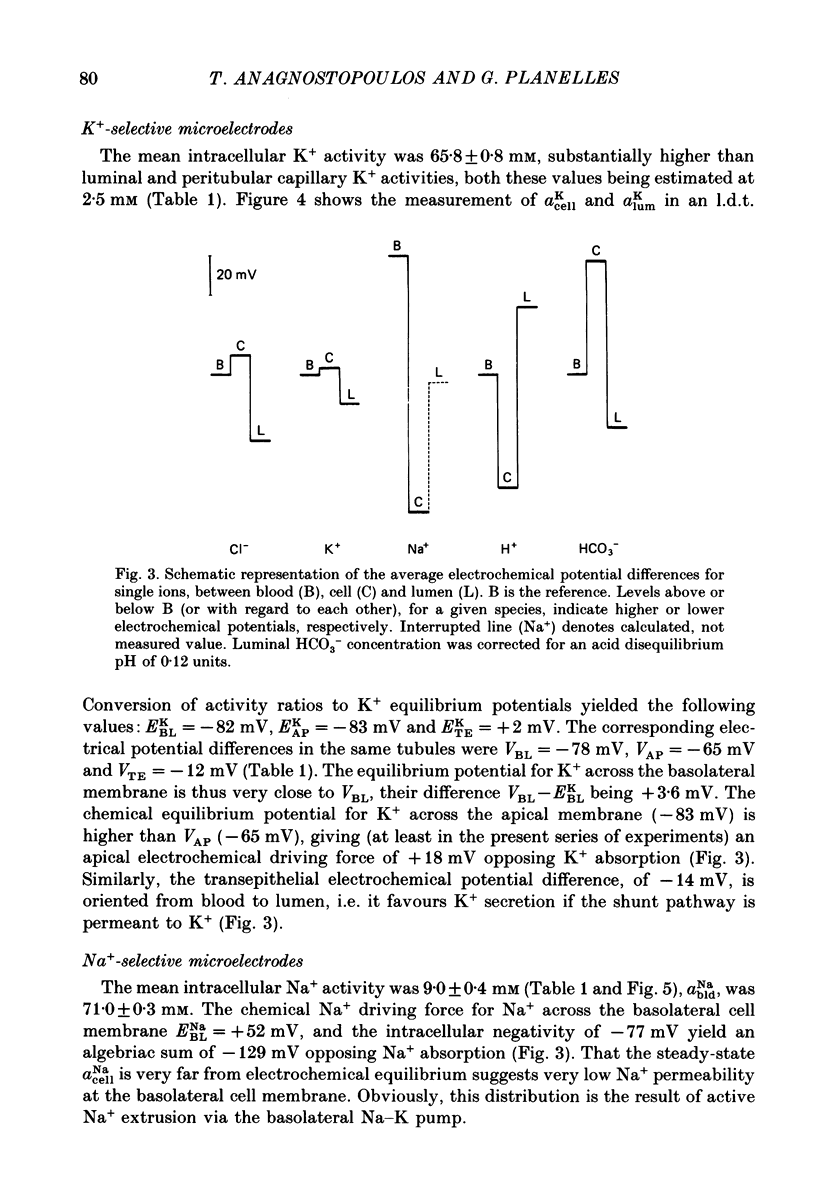
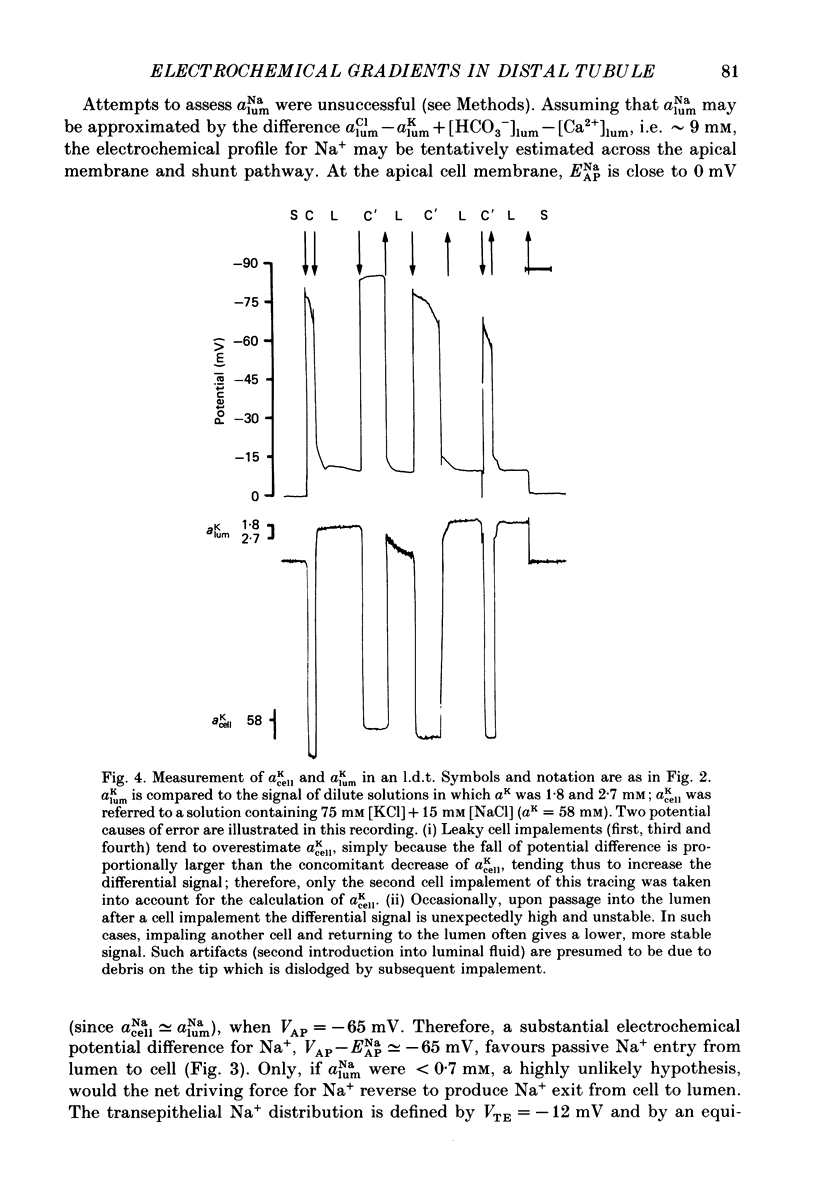
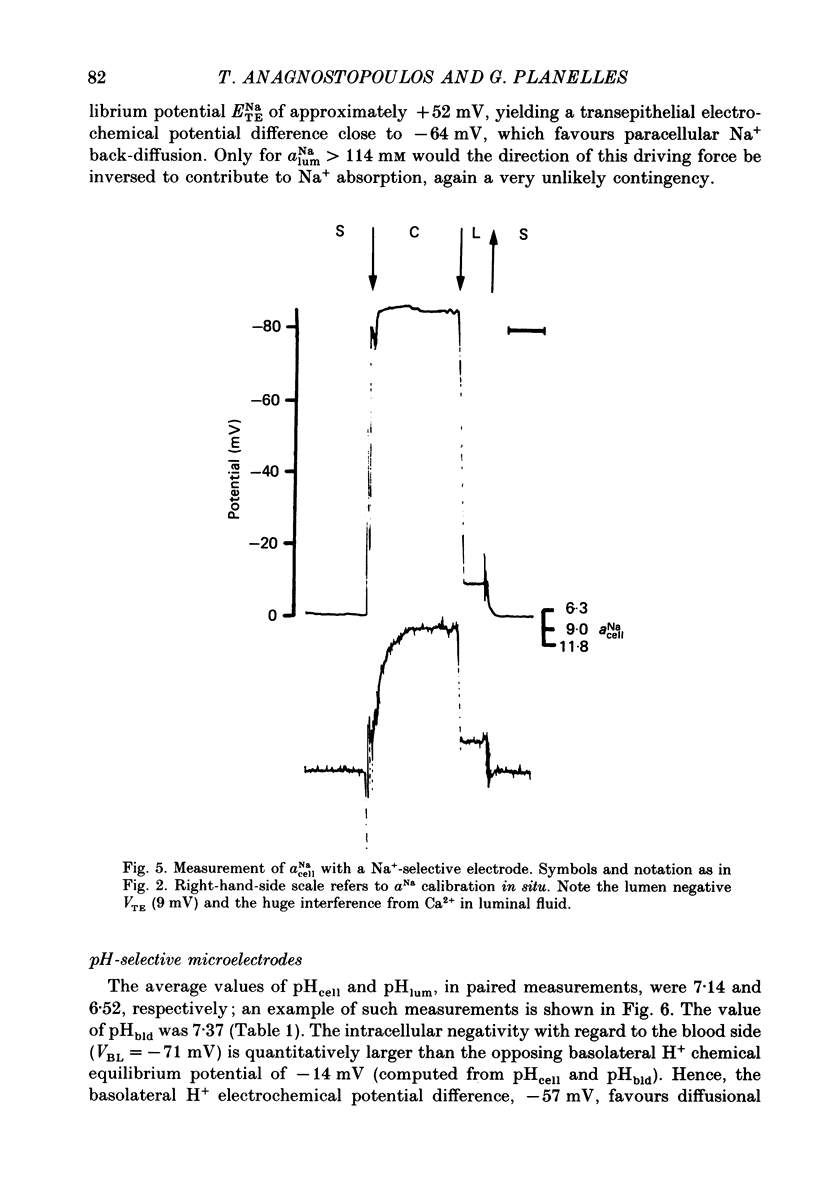
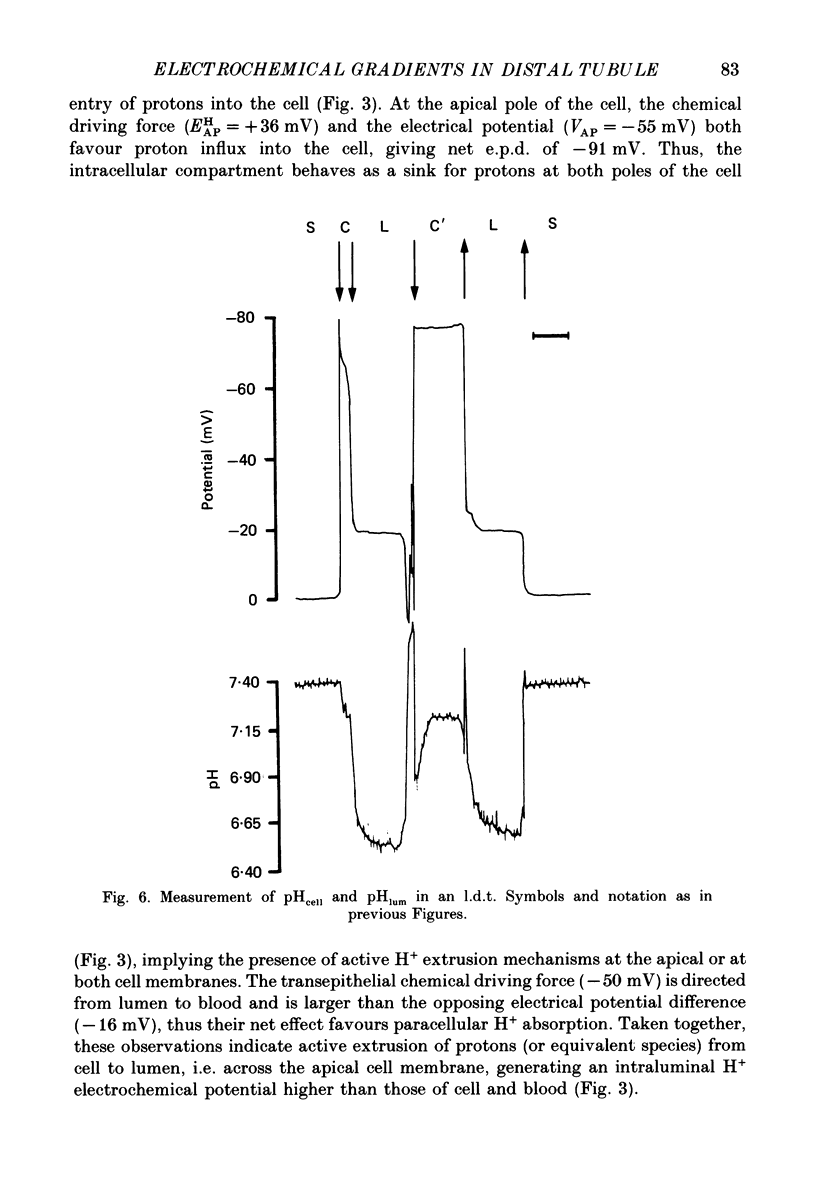
1. Double-barrelled (selective vs. conventional) microelectrodes were used to assess the steady-state activities (a) of the ions Cl-, K+, Na+ and H+ in peritubular blood capillaries (abld) and in cell (acell) and lumen (alum) of the late distal tubule (l.d.t.) of Necturus. 2. a(cell)cl, a(lum)cl and a(bld)cl were 5.5 +/- 0.3, 11.8 +/- 1.0 and 70.5 +/- 0.1 mM, respectively. They were used to compute the chemical potentials for Cl- across the three diffusive barriers of the tissue. Basolateral and apical membrane potentials were -74.3 +/- 1.1 and -60.1 +/- 2.0 mV, respectively (cell negative); the lumen was thus negative with respect to blood, by 13.6 +/- 1.5 mV. The electrochemical potential difference (e.p.d.) for Cl- of 42 mV across the apical membrane opposes Cl- absorption, implying active apical Cl- uptake, since Cl- is known to be absorbed in the l.d.t. Basolateral Cl- exit is favoured by an e.p.d. of 10 mV. 3. a(cell)K, a(lum)K and a(bld)K were 65.8 +/- 0.8, 2.5 +/- 0.1 and 2.5 +/- 0.1 mm, respectively. The electrochemical distribution of K+ indicates that K+ absorption, if present, proceeds against an adverse apical e.p.d. of 18 mV. Basolateral K+ distribution is close to its electrochemical equilibrium, suggesting high K+ permeability at this membrane. 4. a(cell)Na was 9.0 +/- 0.4 mM, a(bld)Na 71.0 +/- 0.3 mM, and a(lum)Na was approximated at about 9 mM. Diffusive Na+ entry from lumen to cell is favoured by an e.p.d. close to 65 mV. Basolateral Na+ exit must be active, since it proceeds against an e.p.d. of 130 mV. 5. Cell, luminal and blood pH were 7.14 +/- 0.03, 6.52 +/- 0.08 and 7.37 +/- 0.04, respectively. The luminal electrochemical potential of H+ is higher than that of cell (by 91 mV) and blood (by 34 mV) indicating that proton secretion into the lumen must be active. 6. The e.p.d. of each ion across the epithelium opposes, by its orientation, the established direction of net transepithelial ion transport, suggesting that the shunt pathway may serve only for back-diffusion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos T. Biionic potentials in the proximal tubule of Necturus kidney. J Physiol. 1973 Sep;233(2):375–394. doi: 10.1113/jphysiol.1973.sp010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTT P. A. Micropuncture study of renal excretion of water, K, Na, and Cl in Necturus. Am J Physiol. 1962 Oct;203:662–666. doi: 10.1152/ajplegacy.1962.203.4.662. [DOI] [PubMed] [Google Scholar]
- Chaillet J. R., Lopes A. G., Boron W. F. Basolateral Na-H exchange in the rabbit cortical collecting tubule. J Gen Physiol. 1985 Dec;86(6):795–812. doi: 10.1085/jgp.86.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Giebisch G., Hansen L. L., Teuscher U., Wiederholt M. Relationship between peritubular membrane potential and net fluid reabsorption in the distal renal tubule of Amphiuma. J Physiol. 1984 Mar;348:115–134. doi: 10.1113/jphysiol.1984.sp015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranti E., Ehrenfeld J., Harvey B. J. Acid secretion through the Rana esculenta skin: involvement of an anion-exchange mechanism at the basolateral membrane. J Physiol. 1986 Sep;378:195–211. doi: 10.1113/jphysiol.1986.sp016214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A., Bouthier M., Anagnostopoulos T. Chloride distribution in the proximal convoluted tubule of Necturus kidney. J Membr Biol. 1981;62(1-2):7–17. doi: 10.1007/BF01870195. [DOI] [PubMed] [Google Scholar]
- Fischer J. L., Husted R. F., Steinmetz P. R. Chloride dependence of the HCO3 exit step in urinary acidification by the turtle bladder. Am J Physiol. 1983 Nov;245(5 Pt 1):F564–F568. doi: 10.1152/ajprenal.1983.245.5.F564. [DOI] [PubMed] [Google Scholar]
- García-Díaz J. F., Klemperer G., Baxendale L. M., Essig A. Cell sodium activity and sodium pump function in frog skin. J Membr Biol. 1986;92(1):37–46. doi: 10.1007/BF01869014. [DOI] [PubMed] [Google Scholar]
- Garland H. O., Henderson I. W., Brown J. A. Micropuncture study of the renal responses of the urodele amphibian Necturus maculosus to injections of arginine vasotocin and an anti-aldosterone compound. J Exp Biol. 1975 Aug;63(1):249–264. doi: 10.1242/jeb.63.1.249. [DOI] [PubMed] [Google Scholar]
- Gluck S., Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest. 1984 Jun;73(6):1704–1710. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Active K transport across rabbit distal colon: relation to Na absorption and Cl secretion. Am J Physiol. 1986 Aug;251(2 Pt 1):C252–C267. doi: 10.1152/ajpcell.1986.251.2.C252. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Kokko J. P., Gross J. B., Jacobson H. R. Electrophysiologic study of the cortical collecting tubule of the rabbit. Kidney Int. 1980 Jan;17(1):74–81. doi: 10.1038/ki.1980.9. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Suzuki Y., Itoi K. Differences in functional properties between the early and the late segments of the distal tubule of amphibian (triturus) kidney. Nihon Jinzo Gakkai Shi. 1981 Jul;23(7):889–896. [PubMed] [Google Scholar]
- Khuri R. N., Agulian S. K., Bogharian K. Electrochemical potentials of chloride in distal renal tubule of the rat. Am J Physiol. 1974 Dec;227(6):1352–1355. doi: 10.1152/ajplegacy.1974.227.6.1352. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Helman S. I. Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol. 1982 May;242(5):F521–F531. doi: 10.1152/ajprenal.1982.242.5.F521. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Taylor A., Windhager E. E. Cytosolic calcium ion activity in epithelial cells of Necturus kidney. Nature. 1980 Oct 30;287(5785):859–861. doi: 10.1038/287859a0. [DOI] [PubMed] [Google Scholar]
- Persson B. E., Persson A. E. Acidification in the distal tubule of the Amphiuma kidney. Acta Physiol Scand. 1983 Mar;117(3):343–349. doi: 10.1111/j.1748-1716.1983.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Planelles G., Kurkdjian A., Anagnostopoulos T. Cell and luminal pH in the proximal tubule of Necturus kidney. Am J Physiol. 1984 Dec;247(6 Pt 2):F932–F938. doi: 10.1152/ajprenal.1984.247.6.F932. [DOI] [PubMed] [Google Scholar]
- Planelles G., Moreau K., Anagnostopoulos T. Reinvestigation of the transepithelial P.D. in the proximal tubule of Necturus kidney. Pflugers Arch. 1983 Jan;396(1):41–48. doi: 10.1007/BF00584696. [DOI] [PubMed] [Google Scholar]
- Reeves R. B. Temperature-induced changes in blood acid-base status: pH and PCO2 in a binary buffer. J Appl Physiol. 1976 May;40(5):752–761. doi: 10.1152/jappl.1976.40.5.752. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y. Intracellular localization of carbonic anhydrase in the frog nephron. Acta Physiol Scand. 1976 Dec;98(4):465–469. doi: 10.1111/j.1748-1716.1976.tb10337.x. [DOI] [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Sansom S. C., Weinman E. J., O'Neil R. G. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984 Aug;247(2 Pt 2):F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Kimura G. Chloride reabsorption by renal proximal tubules of Necturus. J Membr Biol. 1978 Jan 18;38(3):233–254. doi: 10.1007/BF01871924. [DOI] [PubMed] [Google Scholar]
- Star R. A., Burg M. B., Knepper M. A. Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest. 1985 Sep;76(3):1123–1130. doi: 10.1172/JCI112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Stoner L. C. Isolated perfused amphibian renal tubules: the diluting segment. Am J Physiol. 1977 Nov;233(5):F438–F444. doi: 10.1152/ajprenal.1977.233.5.F438. [DOI] [PubMed] [Google Scholar]
- Tago K., Schuster V. L., Stokes J. B. Stimulation of chloride transport by HCO3-CO2 in rabbit cortical collecting tubule. Am J Physiol. 1986 Jul;251(1 Pt 2):F49–F56. doi: 10.1152/ajprenal.1986.251.1.F49. [DOI] [PubMed] [Google Scholar]
- Teulon J., Anagnostopoulos T. The electrical profile of the distal tubule in Triturus kidney. Pflugers Arch. 1982 Nov 1;395(2):138–144. doi: 10.1007/BF00584727. [DOI] [PubMed] [Google Scholar]
- Teulon J., Froissart P., Anagnostopoulos T. Electrochemical profile of K+ and Na+ in the amphibian early distal tubule. Am J Physiol. 1985 Feb;248(2 Pt 2):F266–F271. doi: 10.1152/ajprenal.1985.248.2.F266. [DOI] [PubMed] [Google Scholar]
- Velázquez H., Wright F. S. Effects of diuretic drugs on Na, Cl, and K transport by rat renal distal tubule. Am J Physiol. 1986 Jun;250(6 Pt 2):F1013–F1023. doi: 10.1152/ajprenal.1986.250.6.F1013. [DOI] [PubMed] [Google Scholar]
- White J. F. Omeprazole inhibits H+ secretion by Amphiuma jejunum. Am J Physiol. 1985 Feb;248(2 Pt 1):G256–G259. doi: 10.1152/ajpgi.1985.248.2.G256. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Sullivan W. J., Giebisch G. Potassium and sodium transport across single distal tubules of Amphiuma. J Gen Physiol. 1971 May;57(5):495–525. doi: 10.1085/jgp.57.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


