Abstract
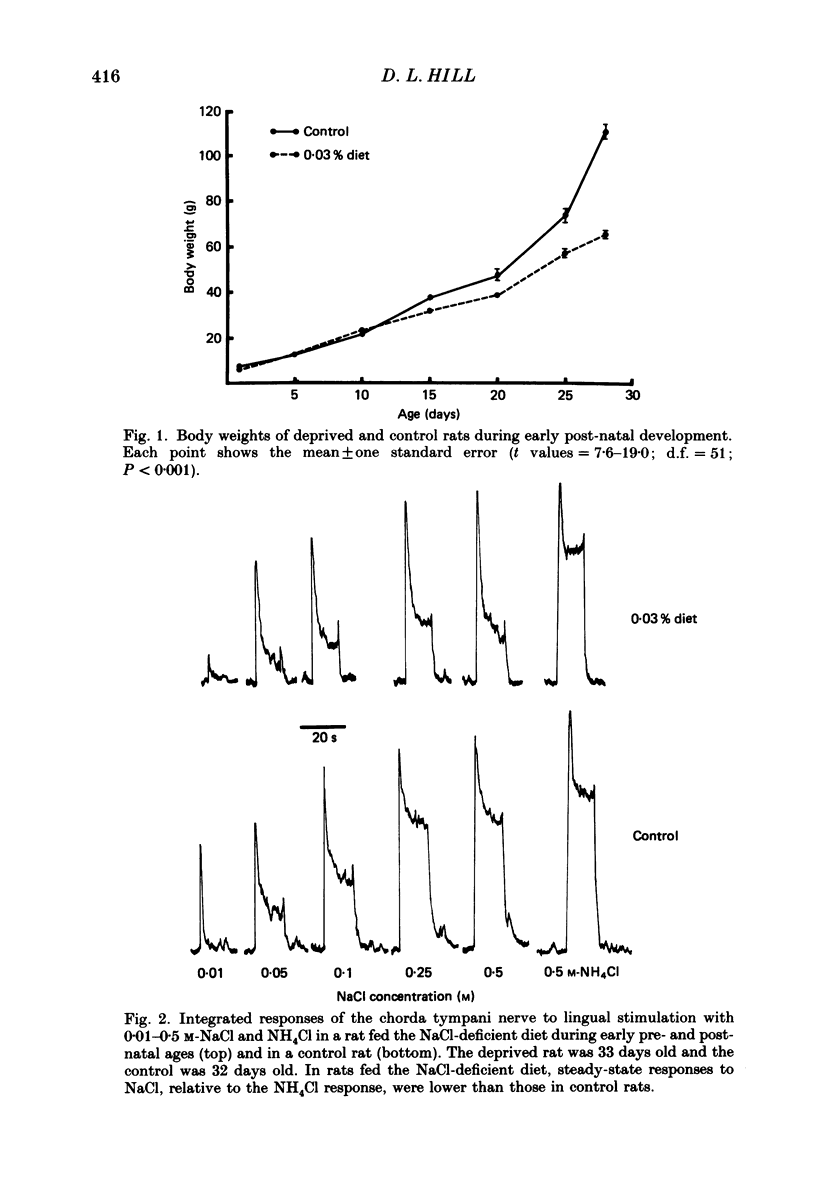
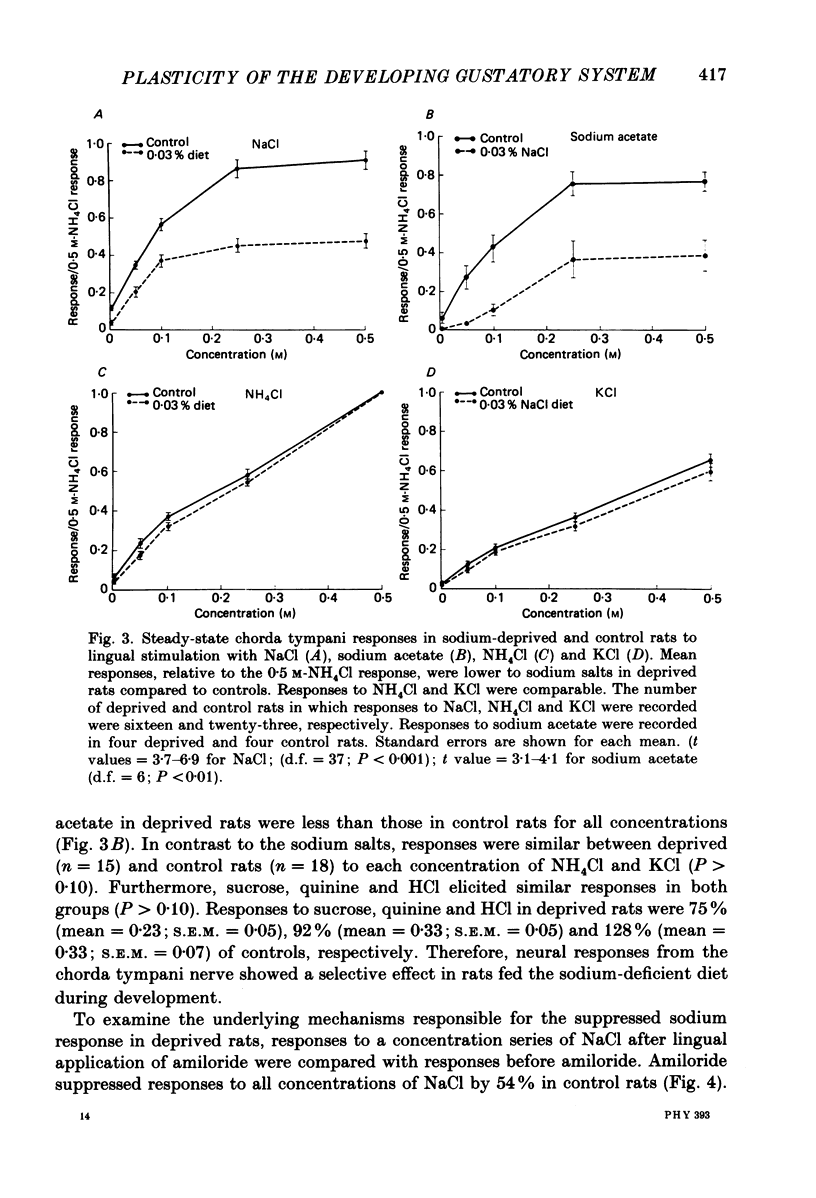
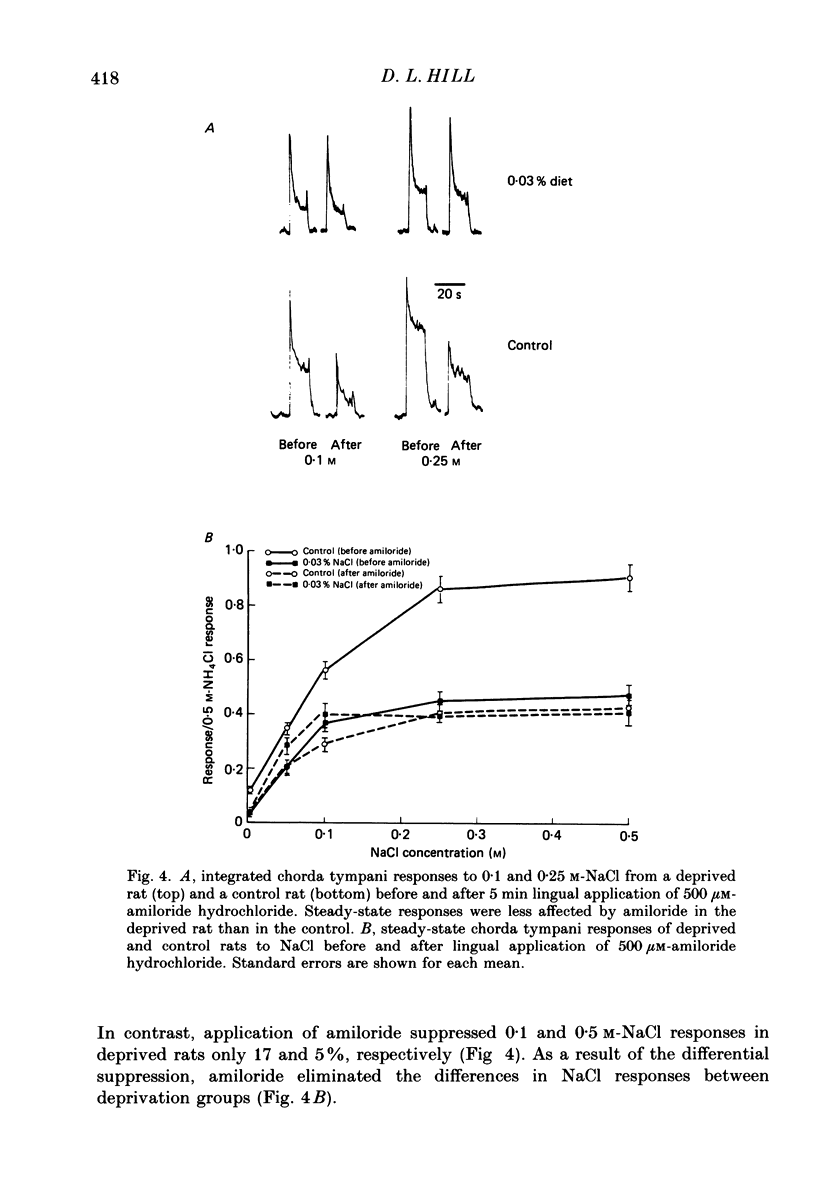
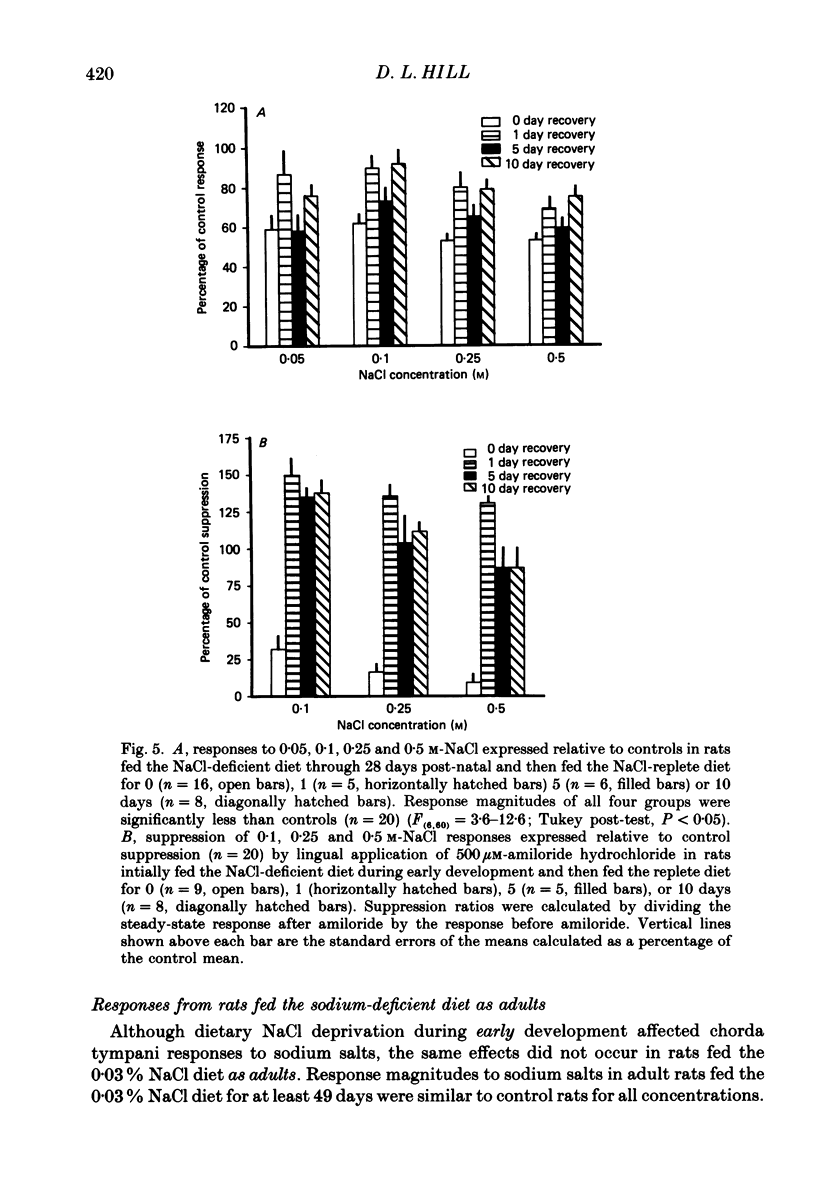
1. Multifibre responses were recorded from the chorda tympani nerve in rats fed either a NaCl-deficient diet or a NaCl-replete diet from 3 days post-conception to at least 28 days post-natal. Responses were also recorded in rats fed the NaCl-deficient diet during early development and then fed the NaCl-replete diet for 1-20 days beginning at 28 days post-natal, and in rats fed the NaCl-deficient diet only as adults. The epithelial sodium transport blocker, amiloride, was used to study the physiological effects of the diet on taste receptor membrane function and to characterize the events involved in recovery of function. 2. Responses to lingual application of sodium salts increased with increasing stimulus concentration; however, response magnitudes were reduced in rats fed the NaCl-deficient diet during early development compared to controls. Responses to non-sodium salts and non-salt stimuli were similar to controls. Amiloride was ineffective in suppressing taste responses to NaCl in deprived rats but effectively suppressed responses in controls by at least 50%. After early-deprived rats were fed a NaCl-replete diet, responses to sodium salts recovered to control levels within 15 days. There was a concomitant decrease in amiloride sensitivity during this period. 3. Rats fed the NaCl-deficient diet from early gestation through adulthood had responses similar to younger deprived rats in that sodium responses were lower than controls. However, rats deprived only as adults were similar to controls. 4. The peripheral gustatory system in developing rats is susceptible to the sodium content of the diet and is 'plastic' in that early effects can be reversed by restricting dietary sodium. Once dietary manipulations are instituted past a sensitive period, however, functional taste responses seem unaffected.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEIDLER L. M., FISHMAN I. Y., HARDIMAN C. W. Species differences in taste responses. Am J Physiol. 1955 May;181(2):235–239. doi: 10.1152/ajplegacy.1955.181.2.235. [DOI] [PubMed] [Google Scholar]
- BEIDLER L. M. Properties of chemoreceptors of tongue of rat. J Neurophysiol. 1953 Nov;16(6):595–607. doi: 10.1152/jn.1953.16.6.595. [DOI] [PubMed] [Google Scholar]
- Beidler L. M., Smallman R. L. Renewal of cells within taste buds. J Cell Biol. 1965 Nov;27(2):263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Contreras R. J., Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979 May;73(5):569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopersmith R., Henderson S. R., Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Brain Res. 1986 Jun;392(1-2):191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Crabbé J. Decreased sensitivity to amiloride of amphibian epithelia treated with aldosterone. Further evidence for an apical hormonal effect. Pflugers Arch. 1980 Jan;383(2):151–158. doi: 10.1007/BF00581876. [DOI] [PubMed] [Google Scholar]
- Doetsch G. S., Erickson R. P. Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rate. J Neurophysiol. 1970 Jul;33(4):490–507. doi: 10.1152/jn.1970.33.4.490. [DOI] [PubMed] [Google Scholar]
- Ferrell M. F., Mistretta C. M., Bradley R. M. Development of chorda tympani taste responses in rat. J Comp Neurol. 1981 May 1;198(1):37–44. doi: 10.1002/cne.901980105. [DOI] [PubMed] [Google Scholar]
- Ganguli M. C., Smith J. D., Hanson L. E. Sodium metabolism and requirements in lactating rats. J Nutr. 1969 Dec;99(4):395–400. doi: 10.1093/jn/99.4.395. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: I. Nature of perceptual deficit caused by embryonic auditory deprivation. J Comp Physiol Psychol. 1975 Jul;89(5):387–399. doi: 10.1037/h0077068. [DOI] [PubMed] [Google Scholar]
- Heck G. L., Mierson S., DeSimone J. A. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984 Jan 27;223(4634):403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Almli C. R. Ontogeny of chorda tympani nerve responses to gustatory stimuli in the rat. Brain Res. 1980 Sep 15;197(1):27–38. doi: 10.1016/0006-8993(80)90432-1. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Bour T. C. Addition of functional amiloride-sensitive components to the receptor membrane: a possible mechanism for altered taste responses during development. Brain Res. 1985 Jun;352(2):310–313. doi: 10.1016/0165-3806(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Bradley R. M., Mistretta C. M. Development of taste responses in rat nucleus of solitary tract. J Neurophysiol. 1983 Oct;50(4):879–895. doi: 10.1152/jn.1983.50.4.879. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Bradley R. M., Mistretta C. M. Development of taste responses in rat nucleus of solitary tract. J Neurophysiol. 1983 Oct;50(4):879–895. doi: 10.1152/jn.1983.50.4.879. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Mistretta C. M., Bradley R. M. Developmental changes in taste response characteristics of rat single chorda tympani fibers. J Neurosci. 1982 Jun;2(6):782–790. doi: 10.1523/JNEUROSCI.02-06-00782.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. L., Mistretta C. M., Bradley R. M. Effects of dietary NaCl deprivation during early development on behavioral and neurophysiological taste responses. Behav Neurosci. 1986 Jun;100(3):390–398. doi: 10.1037//0735-7044.100.3.390. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970 May 15;168(3933):869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Functional architecture of area 17 in normal and monocularly deprived macaque monkeys. Cold Spring Harb Symp Quant Biol. 1976;40:581–589. doi: 10.1101/sqb.1976.040.01.054. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. M., Ostapoff E. M., Rubel E. W. Influence of acoustic experience on the ontogeny of frequency generalization gradients in the chicken. J Exp Psychol Anim Behav Process. 1979 Apr;5(2):97–115. doi: 10.1037//0097-7403.5.2.97. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Li J. H., Lindemann B., Edelman I. S. Aldosterone control of the density of sodium channels in the toad urinary bladder. J Membr Biol. 1982;64(1-2):91–102. doi: 10.1007/BF01870771. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Will P. C., DeLisle R. C., Cortright R. N., Hopfer U. Induction of amiloride-sensitive sodium transport in the intestines by adrenal steroids. Ann N Y Acad Sci. 1981;372:64–78. doi: 10.1111/j.1749-6632.1981.tb15458.x. [DOI] [PubMed] [Google Scholar]
- Will P. C., Lebowitz J. L., Hopfer U. Induction of amiloride-sensitive sodium transport in the rat colon by mineralocorticoids. Am J Physiol. 1980 Apr;238(4):F261–F268. doi: 10.1152/ajprenal.1980.238.4.F261. [DOI] [PubMed] [Google Scholar]
- Yamada T. Chorda tympani responses to gustatory stimuli in developing rats. Jpn J Physiol. 1980;30(4):631–643. doi: 10.2170/jjphysiol.30.631. [DOI] [PubMed] [Google Scholar]


