Abstract
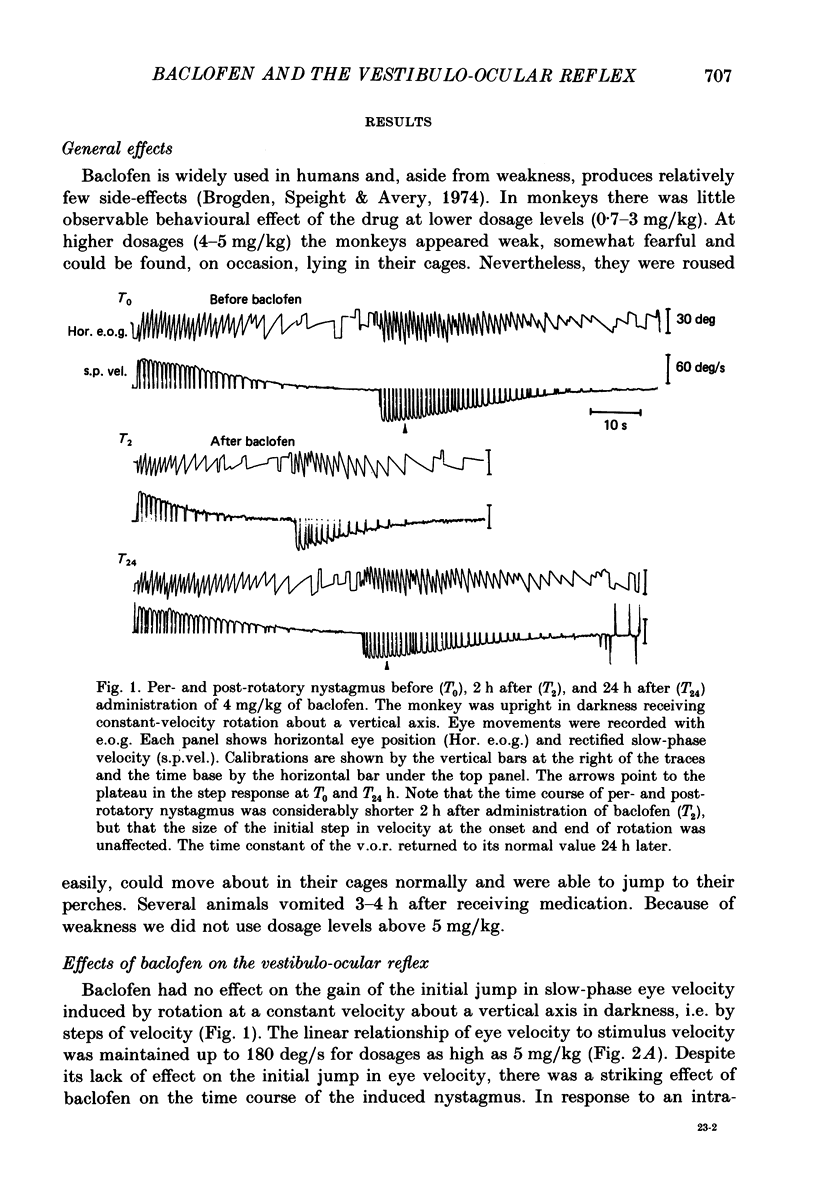
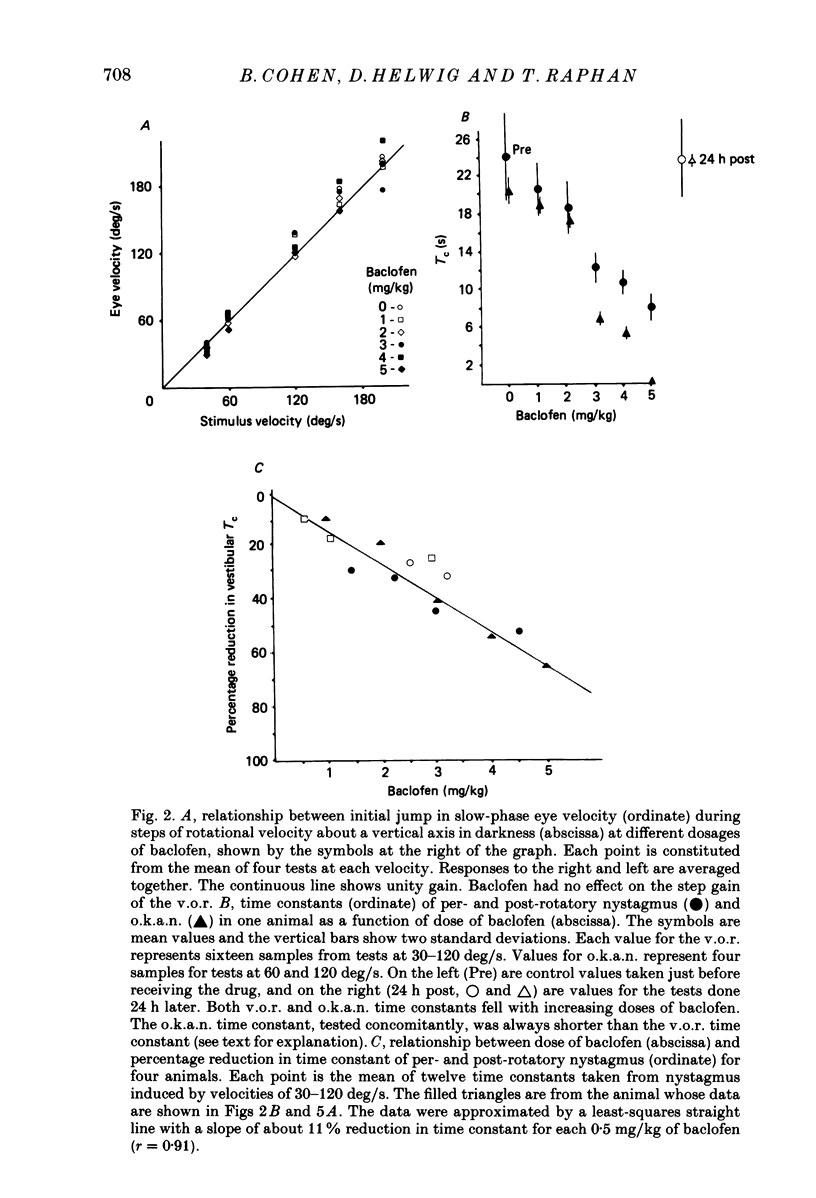
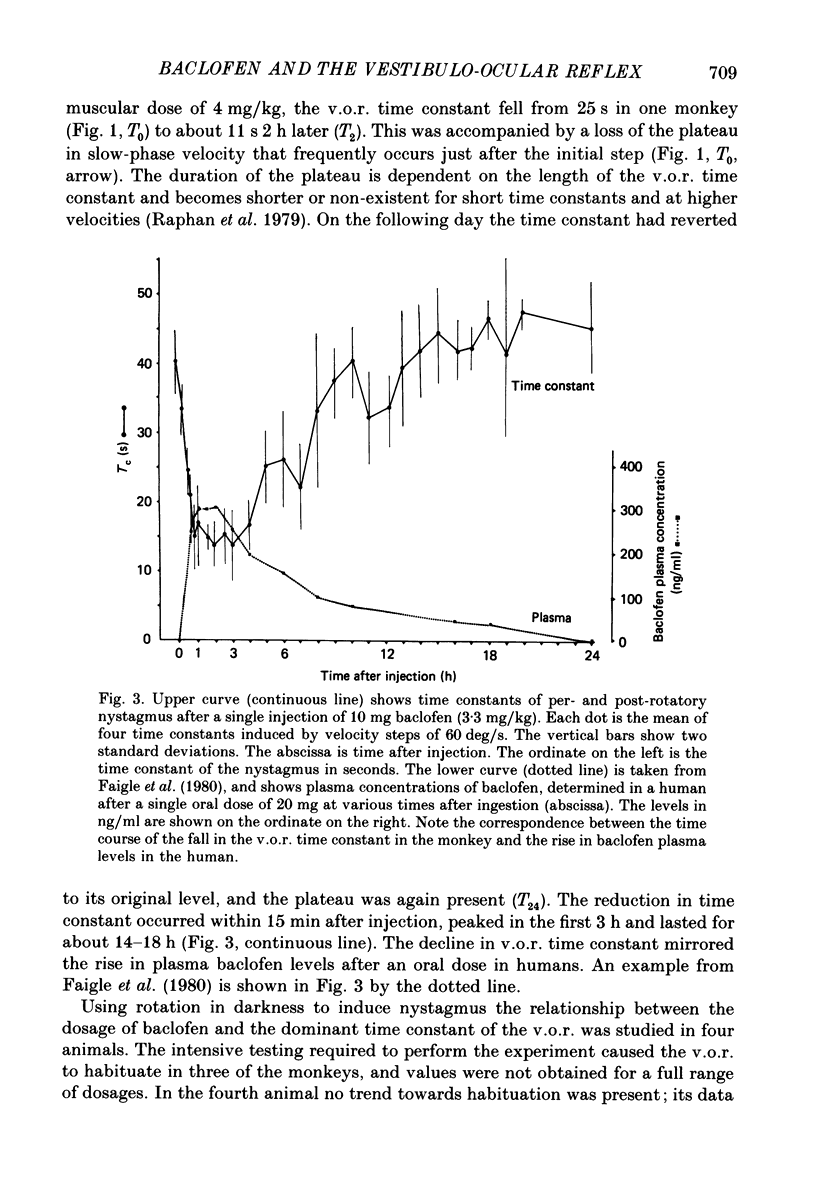
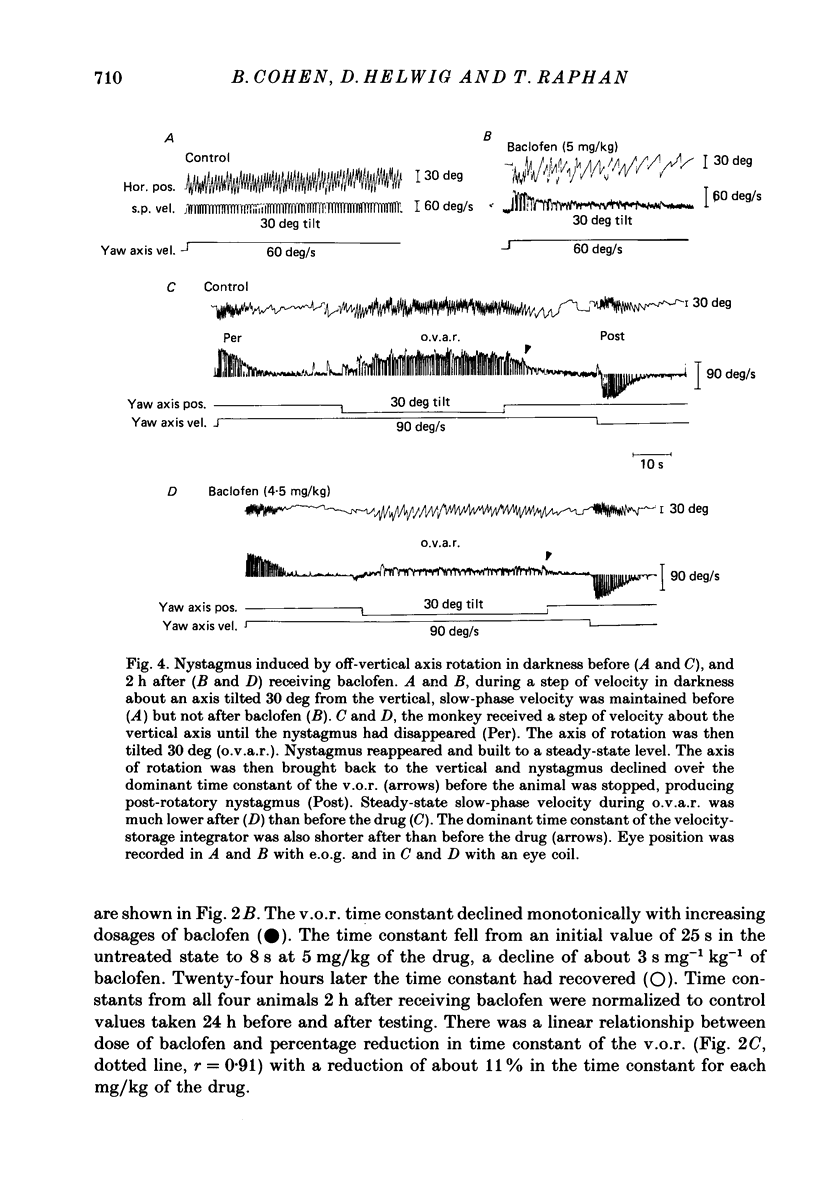
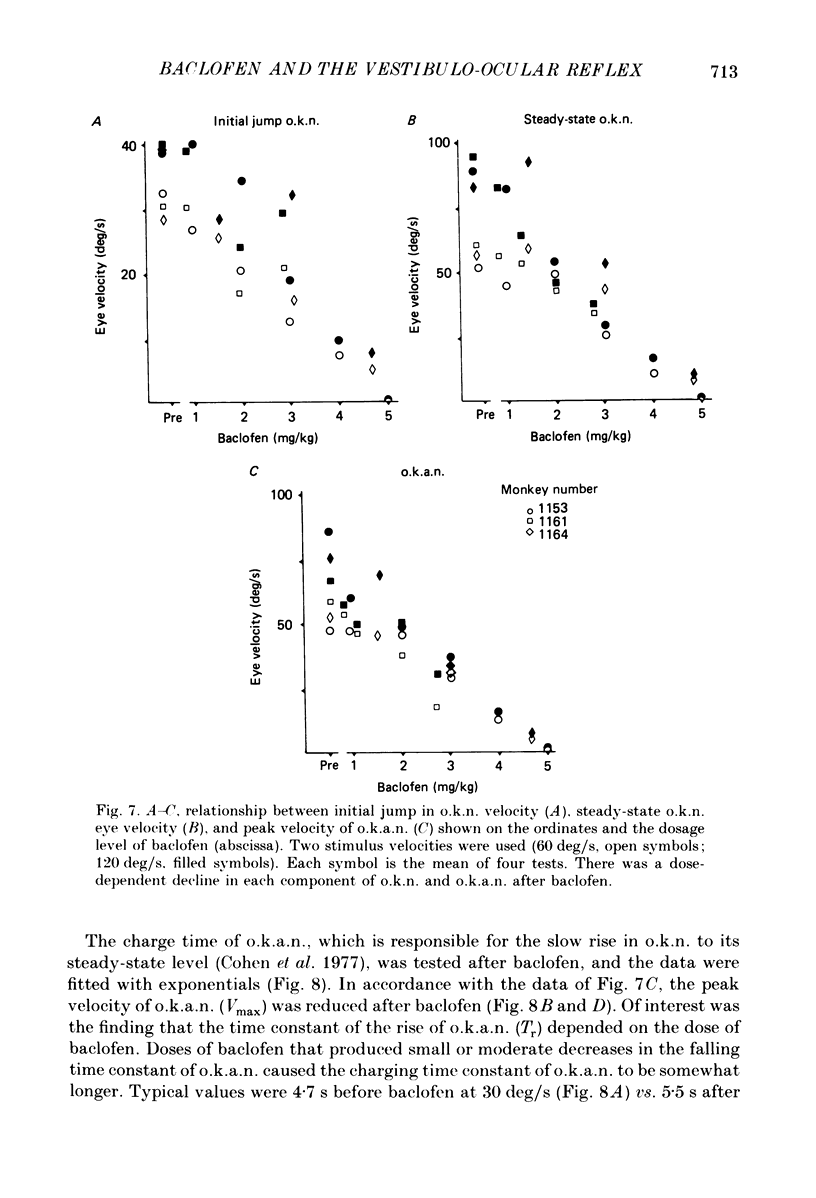
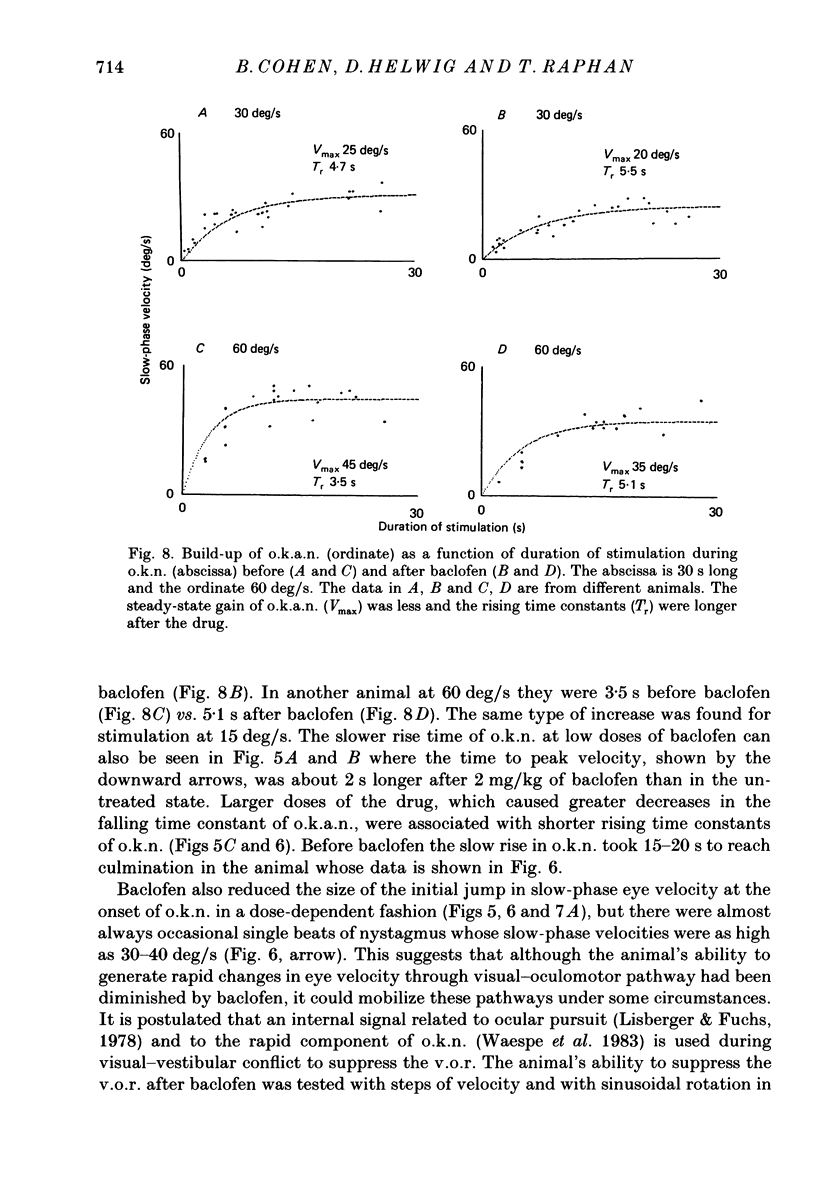
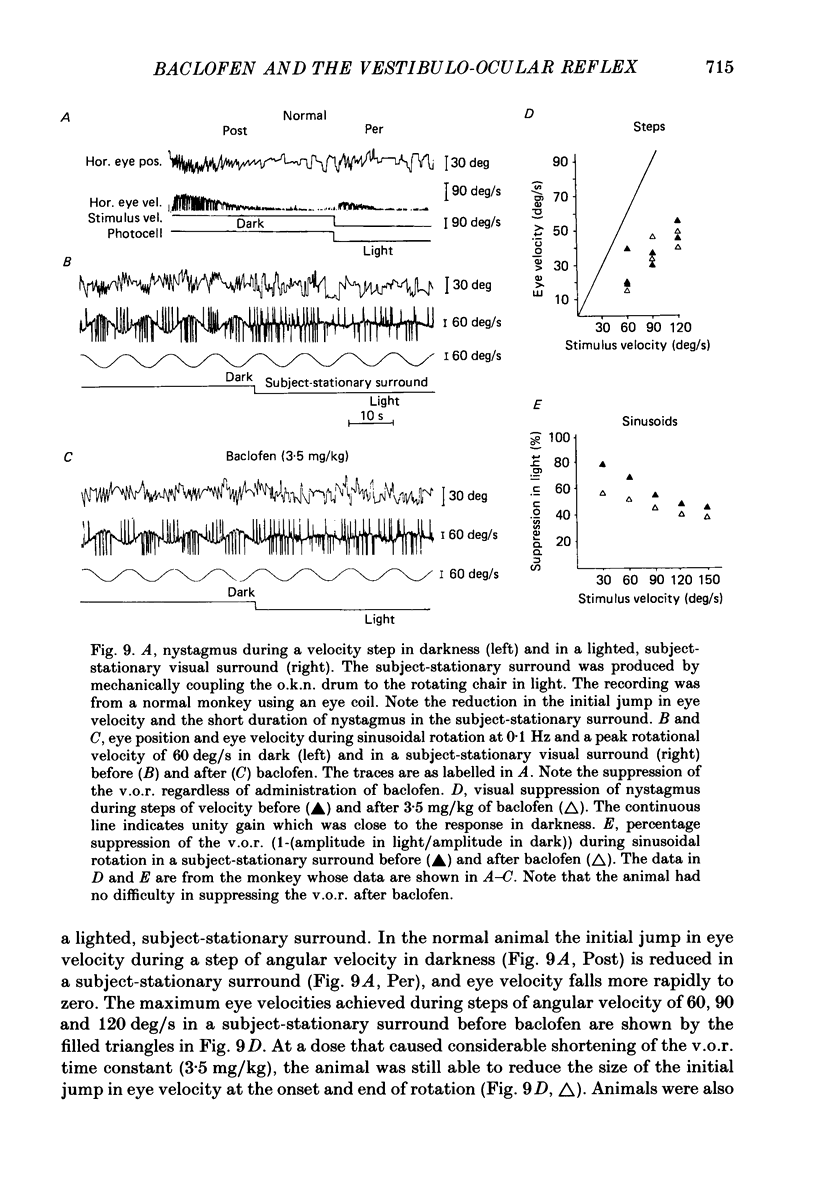
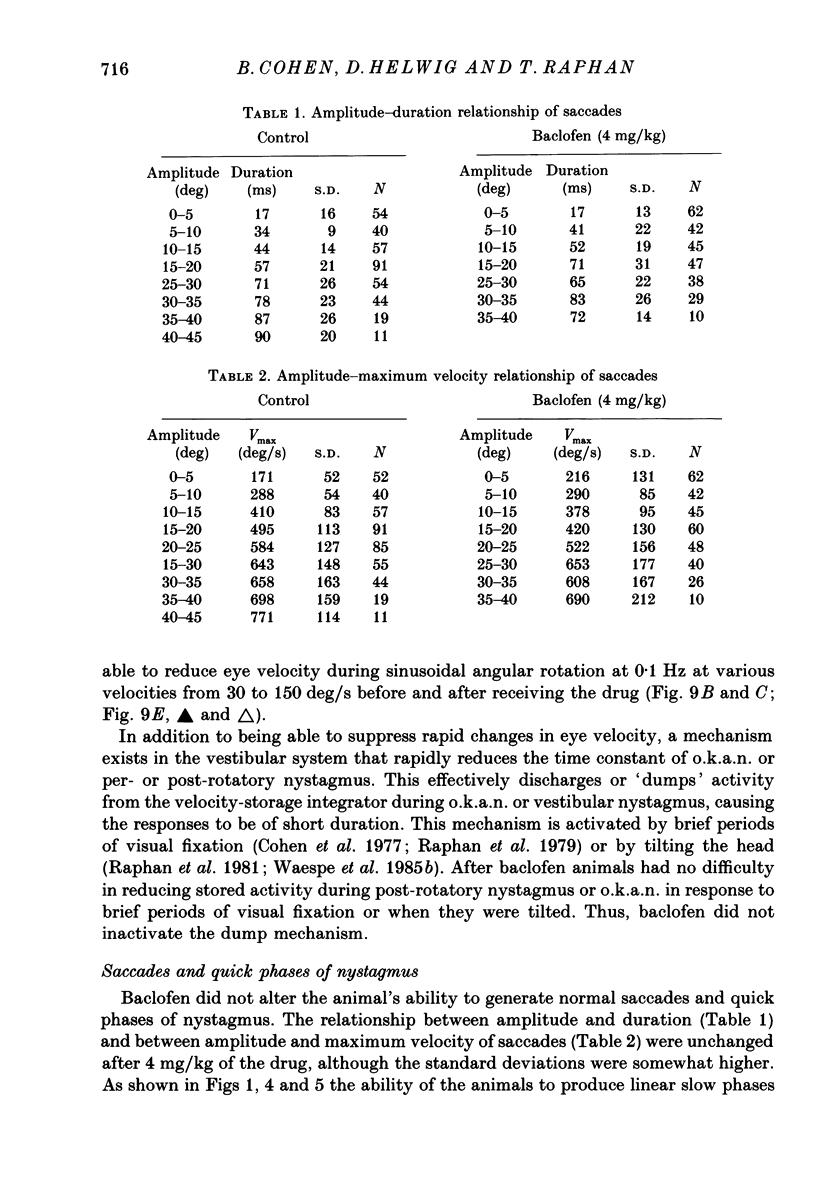
1. Baclofen had a characteristic effect on vestibular and optokinetic nystagmus in rhesus monkeys. Each aspect of nystagmus that is dependent on the velocity-storage mechanism in the vestibulo-ocular reflex (v.o.r.) was altered by the drug: (a) Baclofen reduced the dominant time constant of the v.o.r. in a dose-dependent manner up to 5 mg/kg, the highest dosage used. The alteration in v.o.r. time constant began within 15 min of injection, was maximal between 1 and 4 h, and lasted for 14-18 h. This effect mirrors changes in plasma levels of baclofen after oral doses in humans (Faigle, Keberle & Agen, 1980). (b) Slow-phase velocities of steady-state nystagmus induced by rotation about axes tilted from the vertical (off-vertical axis rotation, o.v.a.r.) were reduced after baclofen and could not be maintained at previous levels. (c) There was a dose-dependent decline in the steady-state gain of optokinetic nystagmus (o.k.n.), and at the highest dosages little o.k.n. was induced. In parallel, the peak velocity and falling time constant of optokinetic after-nystagmus (o.k.a.n.) were reduced. Since baclofen is a GABA agonist, systems utilizing GABA and acting on GABAB receptors appear to produce inhibitory control of velocity storage. 2. The step gain of the v.o.r., measured at the beginning and end of constant-velocity rotation in darkness, was unaffected by baclofen, as were saccades, quick phases of nystagmus, and the ability to hold positions of fixation or to generate linear slow phases of nystagmus. This indicates that it is possible to use baclofen to manipulate the dominant time constant of the v.o.r. and of o.k.a.n. in relative isolation from effects on other oculomotor components. 3. Baclofen caused a dose-dependent reduction in the initial jump in eye velocity at the onset of o.k.n., suggesting that the initial jump is also under inhibitory control of GABAB receptors. However, there were still occasional slow phases with velocities up to 30-40 deg/s after baclofen, and animals were capable of visually suppressing the v.o.r. This indicates that pathways responsible for causing rapid changes in slowphase velocity were capable of functioning, at least intermittently, in the presence of the drug.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson A. J., Bodin M. A. Interaction of linear and angular accelerations on vestibular receptors in man. Aerosp Med. 1966 Feb;37(2):144–154. [PubMed] [Google Scholar]
- Brogden R. N., Speight T. M., Avery G. S. Baclofen: a preliminary report of its pharmacological properties and therapeutic efficacy in spasticity. Drugs. 1974;8(1):1–14. doi: 10.2165/00003495-197408010-00001. [DOI] [PubMed] [Google Scholar]
- Büttner U., Waespe W., Henn V. Duration and direction of optokinetic after-nystagmus as a function of stimulus exposure time in the monkey. Arch Psychiatr Nervenkr (1970) 1976 Dec 30;222(4):281–291. doi: 10.1007/BF00343237. [DOI] [PubMed] [Google Scholar]
- Cohen B., Matsuo V., Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977 Sep;270(2):321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B., Suzuki J. I., Raphan T. Role of the otolith organs in generation of horizontal nystagmus: effects of selective labyrinthine lesions. Brain Res. 1983 Oct 3;276(1):159–164. doi: 10.1016/0006-8993(83)90558-9. [DOI] [PubMed] [Google Scholar]
- Correia M. J., Money K. E. The effect of blockage of all six semicircular canal ducts on nystagmus produced by dynamic linear acceleration in the cat. Acta Otolaryngol. 1970 Jan-Feb;69(1):7–16. doi: 10.3109/00016487009123331. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D. GABA and inhibition of Deiters' neurones. Brain Res. 1970 Sep 29;23(1):117–120. doi: 10.1016/0006-8993(70)90357-4. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Antispasticity drugs: mechanisms of action. Ann Neurol. 1985 Feb;17(2):107–116. doi: 10.1002/ana.410170202. [DOI] [PubMed] [Google Scholar]
- Dememes D., Raymond J., Sans A. Selective retrograde labeling of neurons of the cat vestibular ganglion with [3H]D-aspartate. Brain Res. 1984 Jun 18;304(1):188–191. doi: 10.1016/0006-8993(84)90880-1. [DOI] [PubMed] [Google Scholar]
- Demêmes D., Raymond J. Radioautographic identification of [3H]glutamic acid labeled nerve endings in the cat oculomotor nucleus. Brain Res. 1982 Jan 14;231(2):433–437. doi: 10.1016/0006-8993(82)90379-1. [DOI] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Robinson D. A. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966 May;21(3):1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- GUEDRY F. E., Jr ORIENTATION OF THE ROTATION-AXIS RELATIVE TO GRAVITY: ITS INFLUENCE ON NYSTAGMUS AND THE SENSATION OF ROTATION. Acta Otolaryngol. 1965 Jul-Aug;60:30–48. doi: 10.3109/00016486509126986. [DOI] [PubMed] [Google Scholar]
- Galiana H. L., Flohr H., Jones G. M. A reevaluation of intervestibular nuclear coupling: its role in vestibular compensation. J Neurophysiol. 1984 Feb;51(2):242–259. doi: 10.1152/jn.1984.51.2.242. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Halmagyi G. M., Rudge P., Gresty M. A., Leigh R. J., Zee D. S. Treatment of periodic alternating nystagmus. Ann Neurol. 1980 Dec;8(6):609–611. doi: 10.1002/ana.410080611. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Hailstone M. H., Freeman C. G. Baclofen: stereoselective inhibition of excitant amino acid release. J Pharm Pharmacol. 1980 Mar;32(3):230–231. doi: 10.1111/j.2042-7158.1980.tb12902.x. [DOI] [PubMed] [Google Scholar]
- Judge S. J., Richmond B. J., Chu F. C. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20(6):535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Lanthorn T. H., Cotman C. W. Baclofen selectively inhibits excitatory synaptic transmission in the hippocampus. Brain Res. 1981 Nov 23;225(1):171–178. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- Leigh R. J., Robinson D. A., Zee D. S. A hypothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann N Y Acad Sci. 1981;374:619–635. doi: 10.1111/j.1749-6632.1981.tb30906.x. [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Fuchs A. F. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978 May;41(3):733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Miles F. A., Fuller J. H. Visual tracking and the primate flocculus. Science. 1975 Sep 19;189(4207):1000–1002. doi: 10.1126/science.1083068. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Obata K., Highstein S. M. Blocking by picrotoxin of both vestibular inhibition and GABA action on rabbit oculomotor neurones. Brain Res. 1970 Mar 17;18(3):538–541. doi: 10.1016/0006-8993(70)90136-8. [DOI] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release and metabolism in slices of guinea pig cerebral cortex. J Neurochem. 1979 Jan;32(1):103–109. doi: 10.1111/j.1471-4159.1979.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Precht W., Baker R., Okada Y. Evidence for GABA as the synaptic transmitter of the inhibitory vestibulo-ocular pathway. Exp Brain Res. 1973 Nov 29;18(4):415–428. doi: 10.1007/BF00239109. [DOI] [PubMed] [Google Scholar]
- Price G. W., Wilkin G. P., Turnbull M. J., Bowery N. G. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984 Jan 5;307(5946):71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- ROBINSON D. A. A METHOD OF MEASURING EYE MOVEMENT USING A SCLERAL SEARCH COIL IN A MAGNETIC FIELD. IEEE Trans Biomed Eng. 1963 Oct;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Raphan T., Cohen B., Henn V. Effects of gravity on rotatory nystagmus in monkeys. Ann N Y Acad Sci. 1981;374:44–55. doi: 10.1111/j.1749-6632.1981.tb30859.x. [DOI] [PubMed] [Google Scholar]
- Raphan T., Cohen B., Suzuki J., Henn V. Nystagmus generated by sinusoidal pitch while rotating. Brain Res. 1983 Oct 3;276(1):165–172. doi: 10.1016/0006-8993(83)90559-0. [DOI] [PubMed] [Google Scholar]
- Raphan T., Cohen B. Velocity storage and the ocular response to multidimensional vestibular stimuli. Rev Oculomot Res. 1985;1:123–143. [PubMed] [Google Scholar]
- Raphan T., Matsuo V., Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res. 1979 Apr 2;35(2):229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Raymond J., Nieoullon A., Demêmes D., Sans A. Evidence for glutamate as a neurotransmitter in the cat vestibular nerve: radioautographic and biochemical studies. Exp Brain Res. 1984;56(3):523–531. doi: 10.1007/BF00237993. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950 Nov;13(6):395–407. doi: 10.1152/jn.1950.13.6.395. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Gallager D. W. The GABA-ergic system: a locus of benzodiazepine action. Annu Rev Neurosci. 1985;8:21–44. doi: 10.1146/annurev.ne.08.030185.000321. [DOI] [PubMed] [Google Scholar]
- Waespe W., Cohen B., Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985 Apr 12;228(4696):199–202. doi: 10.1126/science.3871968. [DOI] [PubMed] [Google Scholar]
- Waespe W., Cohen B., Raphan T. Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res. 1983;50(1):9–33. doi: 10.1007/BF00238229. [DOI] [PubMed] [Google Scholar]
- Waespe W., Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res. 1977 Apr 21;27(5):523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- Waespe W., Henn V. Visual-vestibular interaction in the flocculus of the alert monkey. II. Purkinje cell activity. Exp Brain Res. 1981;43(3-4):349–360. doi: 10.1007/BF00238377. [DOI] [PubMed] [Google Scholar]
- Waespe W., Rudinger D., Wolfensberger M. Purkinje cell activity in the flocculus of vestibular neurectomized and normal monkeys during optokinetic nystagmus (OKN) and smooth pursuit eye movements. Exp Brain Res. 1985;60(2):243–262. doi: 10.1007/BF00235919. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yamazaki A., Butler P. H., Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981 Oct;46(4):878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- de Jong J. M., Cohen B., Matsuo V., Uemura T. Midsagittal pontomedullary brain stem section: effects on ocular adduction and nystagmus. Exp Neurol. 1980 Jun;68(3):420–442. doi: 10.1016/0014-4886(80)90098-9. [DOI] [PubMed] [Google Scholar]


