Abstract
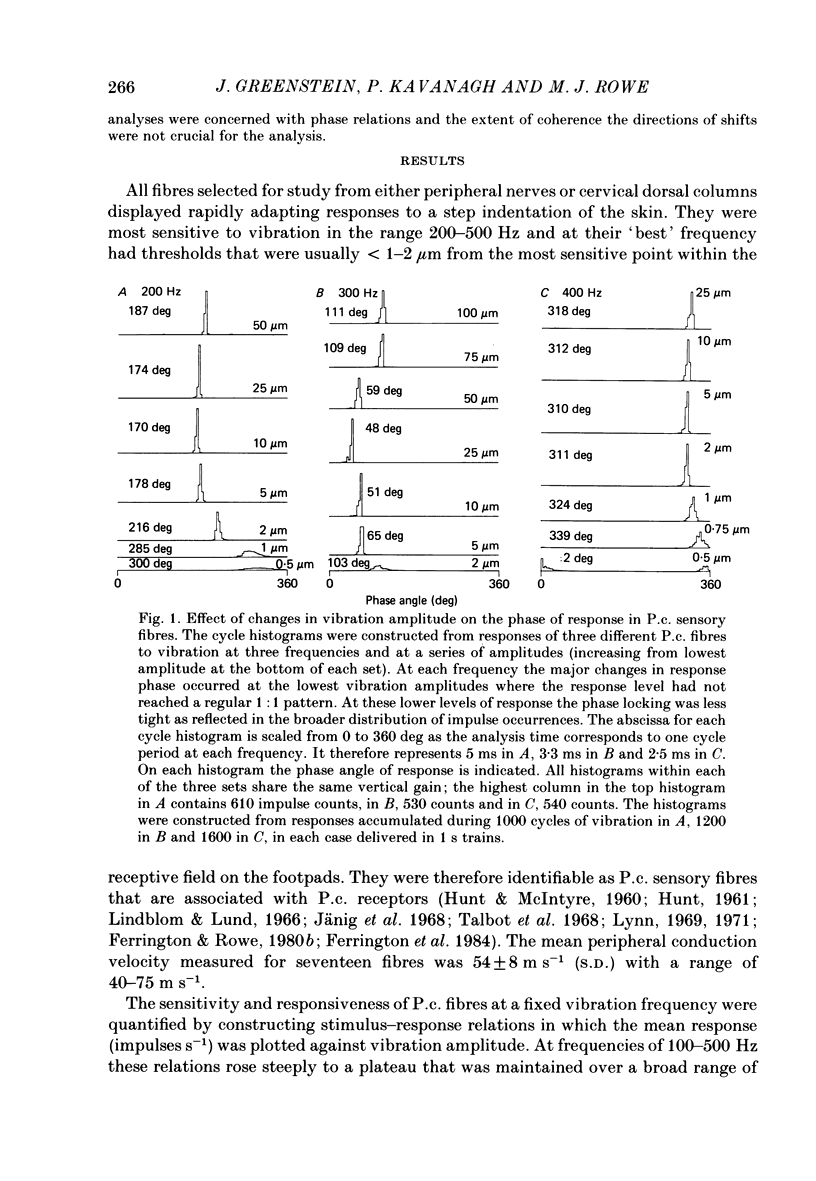
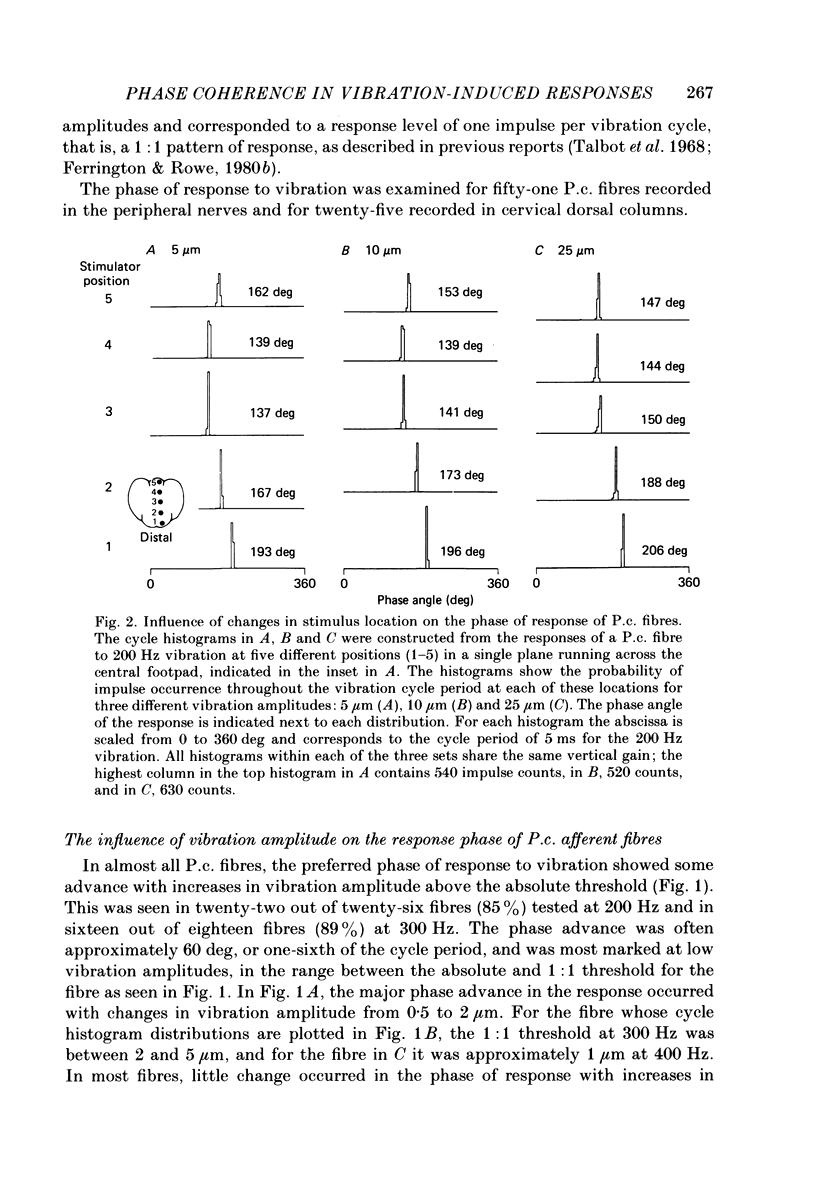
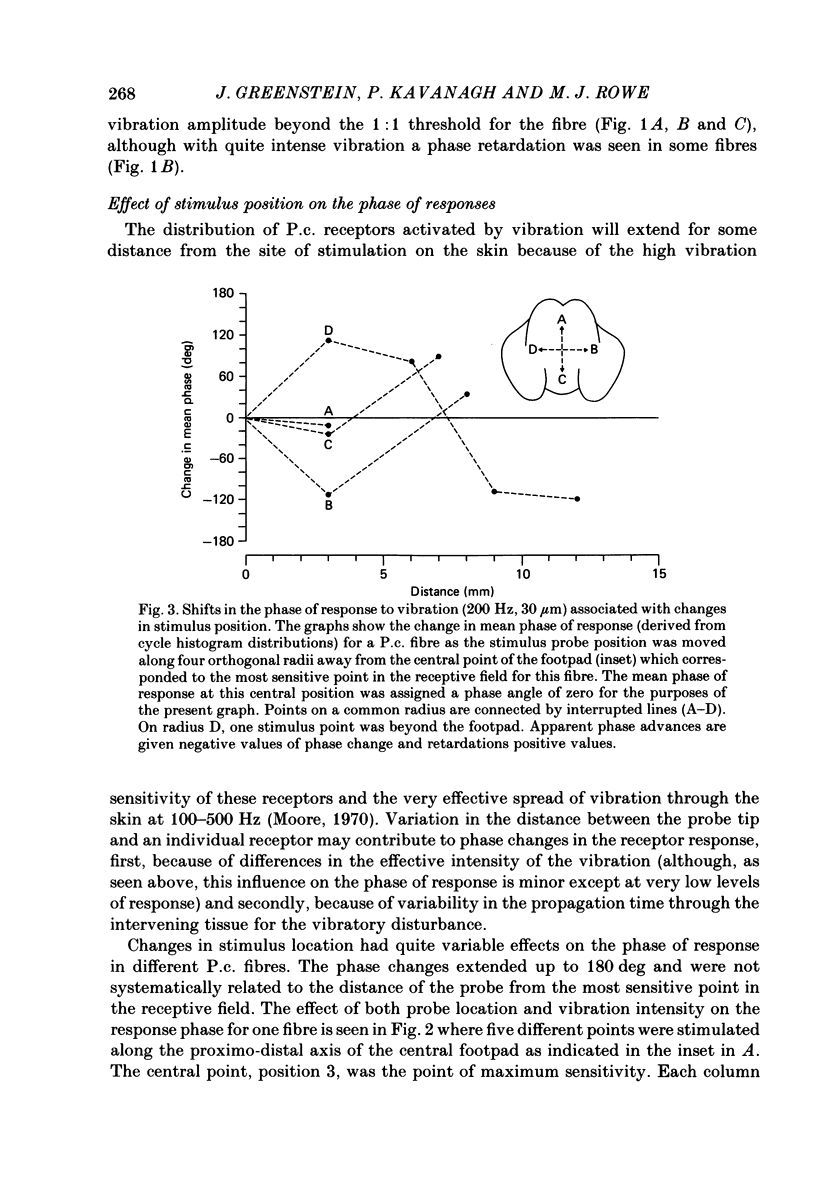
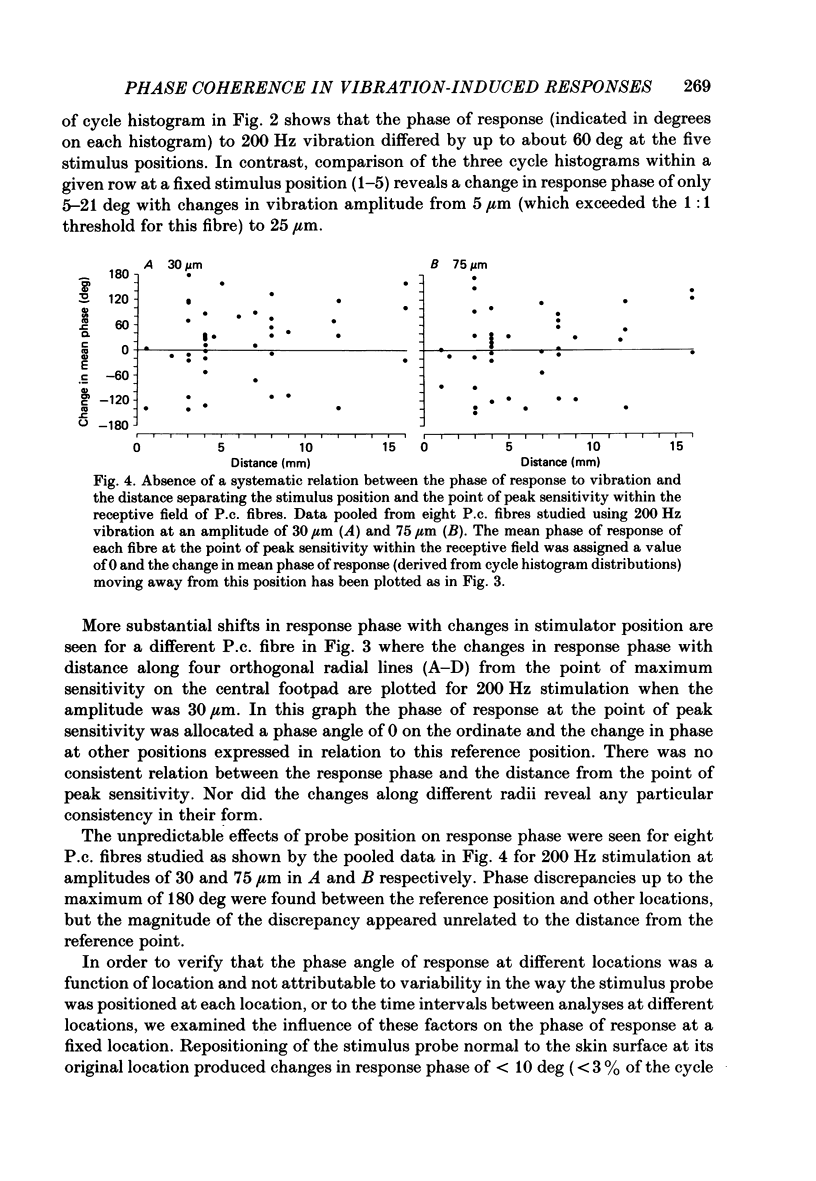
1. In pentobarbitone-anaesthetized cats, responses were recorded in peripheral nerves or cervical dorsal columns from sensory fibres associated with Pacinian corpuscle (P.c.) receptors in the forelimb footpads. Factors affecting the phase of response to cutaneous vibration in individual P.c. fibres, and the extent of phase coherence in the responses of different P.c. fibres were examined when sinusoidal vibratory stimuli at 100-400 Hz were delivered using a 1 mm diameter probe. 2. Increases in vibration amplitude from the absolute to the 1:1 threshold for the P.c. fibre led to phase advances in the response, often of about 60 deg, in over 85% of fibres tested at 200 and 300 Hz, but further increases had little effect. 3. Variations in stimulus position within the receptive field led to unpredictable changes in the response phase that ranged from minimal change to shifts of 180 deg. As the response phase was unrelated to the distance from the point of peak sensitivity it is likely that at high vibration frequencies (greater than or equal to 100 Hz) the recruited population of P.c. fibres will respond over the whole range of phase angles. 4. The calculated phase of spike initiation in different pairs of P.c. fibres that shared coincident points of best sensitivity on the skin ranged from near synchrony to maximum asynchrony indicating that there is little phase coherence even in the subpopulation of somatotopically related P.c. fibres recruited by high-frequency cutaneous vibration. 5. Paired recordings from P.c. fibres within the cervical dorsal columns revealed a broad range of phase discrepancies in the responses of P.c. fibres to vibration at 200 and 300 Hz. 6. Several hypotheses are considered to explain the known presence of phase-locked responses to high-frequency (greater than or equal to 100 Hz) vibration in the central neurones of dorsal column nuclei.
Full text
PDF


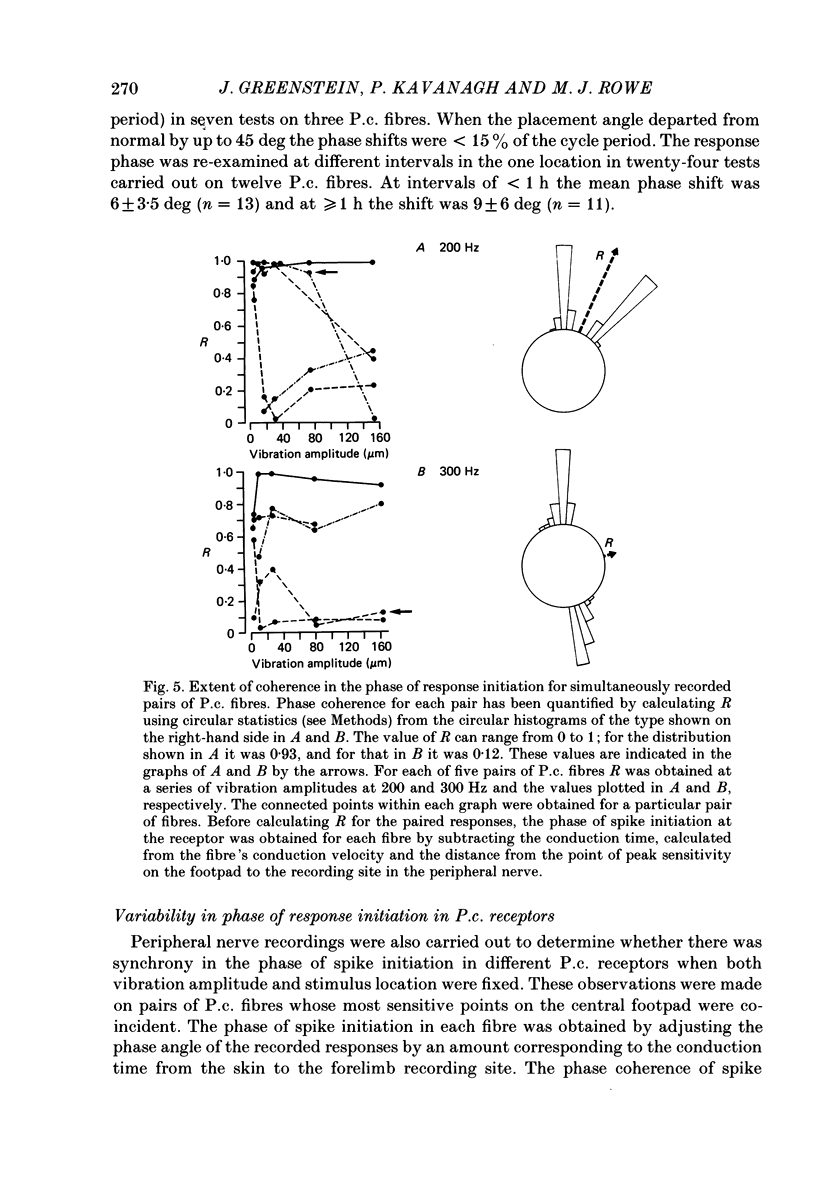
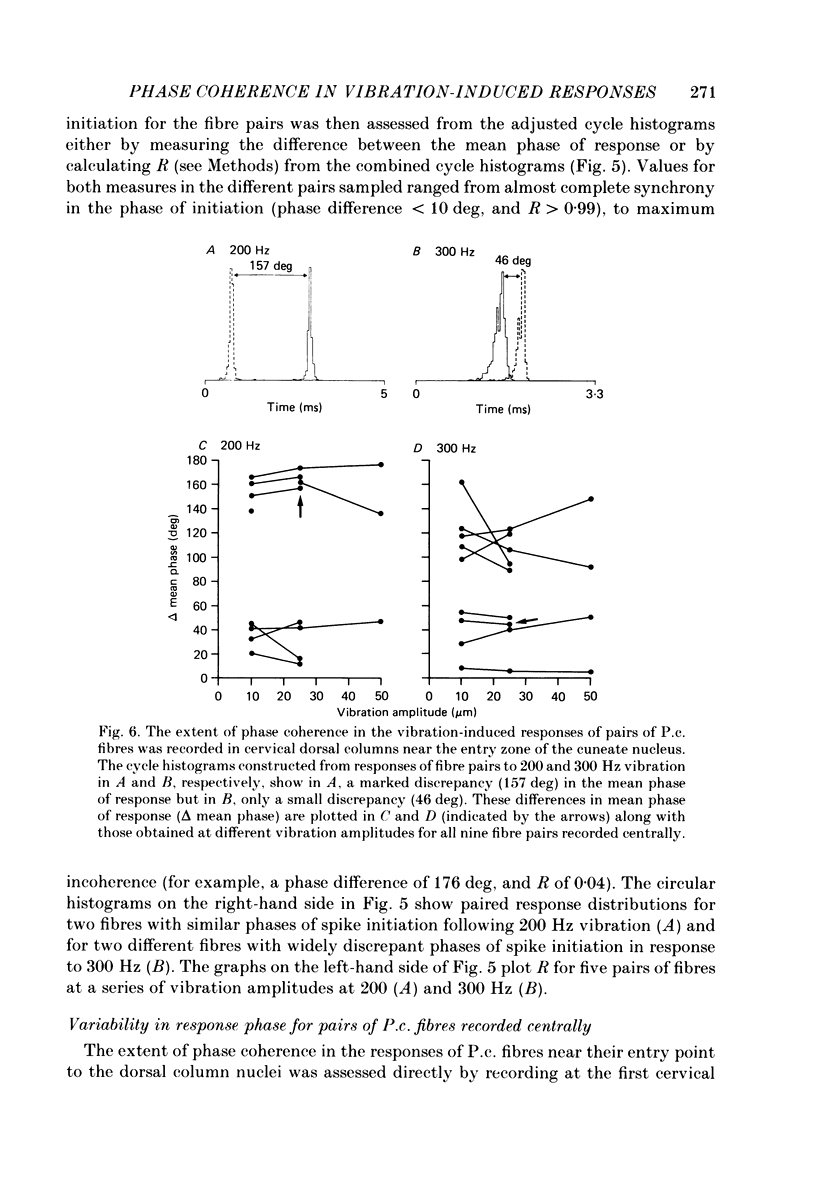




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R. E., Ferrington D. G., Rowe M. Tactile neuron classes within second somatosensory area (SII) of cat cerebral cortex. J Neurophysiol. 1980 Feb;43(2):292–309. doi: 10.1152/jn.1980.43.2.292. [DOI] [PubMed] [Google Scholar]
- Bledsoe S. C., Jr, Rupert A. L., Moushegian G. Response characteristics of cochlear nucleus neurons to 500-Hz tones and noise: findings relating to frequency-following potentials. J Neurophysiol. 1982 Jan;47(1):113–127. doi: 10.1152/jn.1982.47.1.113. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J Physiol. 1983 Apr;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K. M., Ferrington D. G., Rowe M. J. Tactile sensory coding during development: signaling capacities of neurons in kitten dorsal column nuclei. J Neurophysiol. 1984 Jul;52(1):86–98. doi: 10.1152/jn.1984.52.1.86. [DOI] [PubMed] [Google Scholar]
- Douglas P. R., Ferrington D. G., Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978 Dec;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Hora M. O., Rowe M. J. Functional maturation of tactile sensory fibers in the kitten. J Neurophysiol. 1984 Jul;52(1):74–85. doi: 10.1152/jn.1984.52.1.74. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Horniblow S., Rowe M. J. Temporal patterning in the responses of gracile and cuneate neurones in the cat to cutaneous vibration. J Physiol. 1987 May;386:277–291. doi: 10.1113/jphysiol.1987.sp016534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Functional capacities of tactile afferent fibres in neonatal kittens. J Physiol. 1980 Oct;307:335–353. doi: 10.1113/jphysiol.1980.sp013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Specificity of connections and tactile coding capacities in cuneate nucleus of the neonatal kitten. J Neurophysiol. 1982 Apr;47(4):622–640. doi: 10.1152/jn.1982.47.4.622. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Actions of single sensory fibres on cat dorsal column nuclei neurones: vibratory signalling in a one-to-one linkage. J Physiol. 1987 May;386:293–309. doi: 10.1113/jphysiol.1987.sp016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Integrative processing of vibratory information in cat dorsal column nuclei neurones driven by identified sensory fibres. J Physiol. 1987 May;386:311–331. doi: 10.1113/jphysiol.1987.sp016536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. Differential contributions to coding of cutaneous vibratory information by cortical somatosensory areas I and II. J Neurophysiol. 1980 Feb;43(2):310–331. doi: 10.1152/jn.1980.43.2.310. [DOI] [PubMed] [Google Scholar]
- Goff G. D. Differential discrimination of frequency of cutaneous mechanical vibration. J Exp Psychol. 1967 Jun;74(2):294–299. doi: 10.1037/h0024561. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Brown P. B. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969 Jul;32(4):613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. O. Reconstruction of population response to a vibratory stimulus in quickly adapting mechanoreceptive afferent fiber population innervating glabrous skin of the monkey. J Neurophysiol. 1974 Jan;37(1):48–72. doi: 10.1152/jn.1974.37.1.48. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Lavine R. A. Phase-locking in response of single neurons in cochlear nucler complex of the cat to low-frequency tonal stimuli. J Neurophysiol. 1971 May;34(3):467–483. doi: 10.1152/jn.1971.34.3.467. [DOI] [PubMed] [Google Scholar]
- Lindblom U., Lund L. The discharge from vibration-sensitive receptors in the monkey foot. Exp Neurol. 1966 Aug;15(4):401–417. doi: 10.1016/0014-4886(66)90138-5. [DOI] [PubMed] [Google Scholar]
- Lynn B. The form and distribution of the receptive fields of Pacinian corpuscles found in and around the cat's large foot pad. J Physiol. 1971 Sep;217(3):755–771. doi: 10.1113/jphysiol.1971.sp009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B. The nature and location of certain phasic mechanoreceptors in the cat's foot. J Physiol. 1969 May;201(3):765–773. doi: 10.1113/jphysiol.1969.sp008786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B. The view from within: pathways to the study of perception. Johns Hopkins Med J. 1975 Mar;136(3):109–131. [PubMed] [Google Scholar]
- Rose J. E., Brugge J. F., Anderson D. J., Hind J. E. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967 Jul;30(4):769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Rothenberg M., Verrillo R. T., Zahorian S. A., Brachman M. L., Bolanowski S. J., Jr Vibrotactile frequency for encoding a speech parameter. J Acoust Soc Am. 1977 Oct;62(4):1003–1012. doi: 10.1121/1.381610. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]


