Abstract
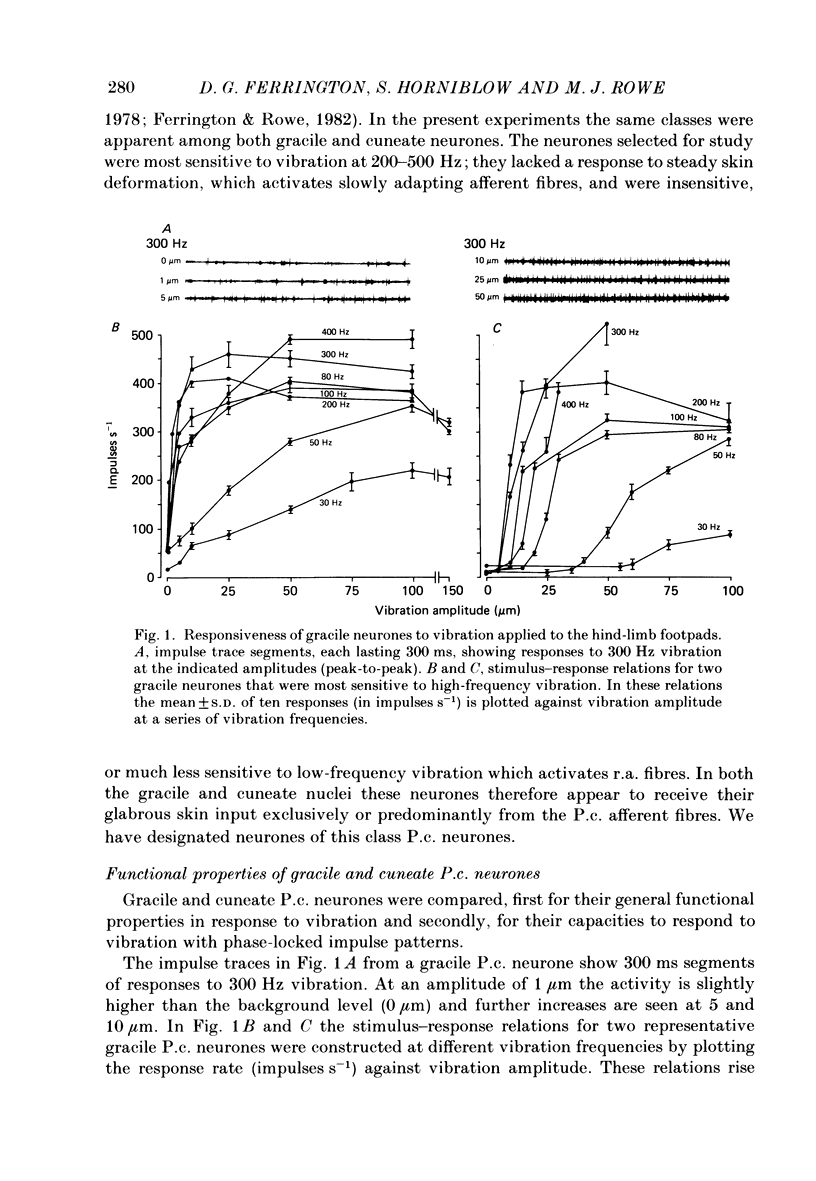
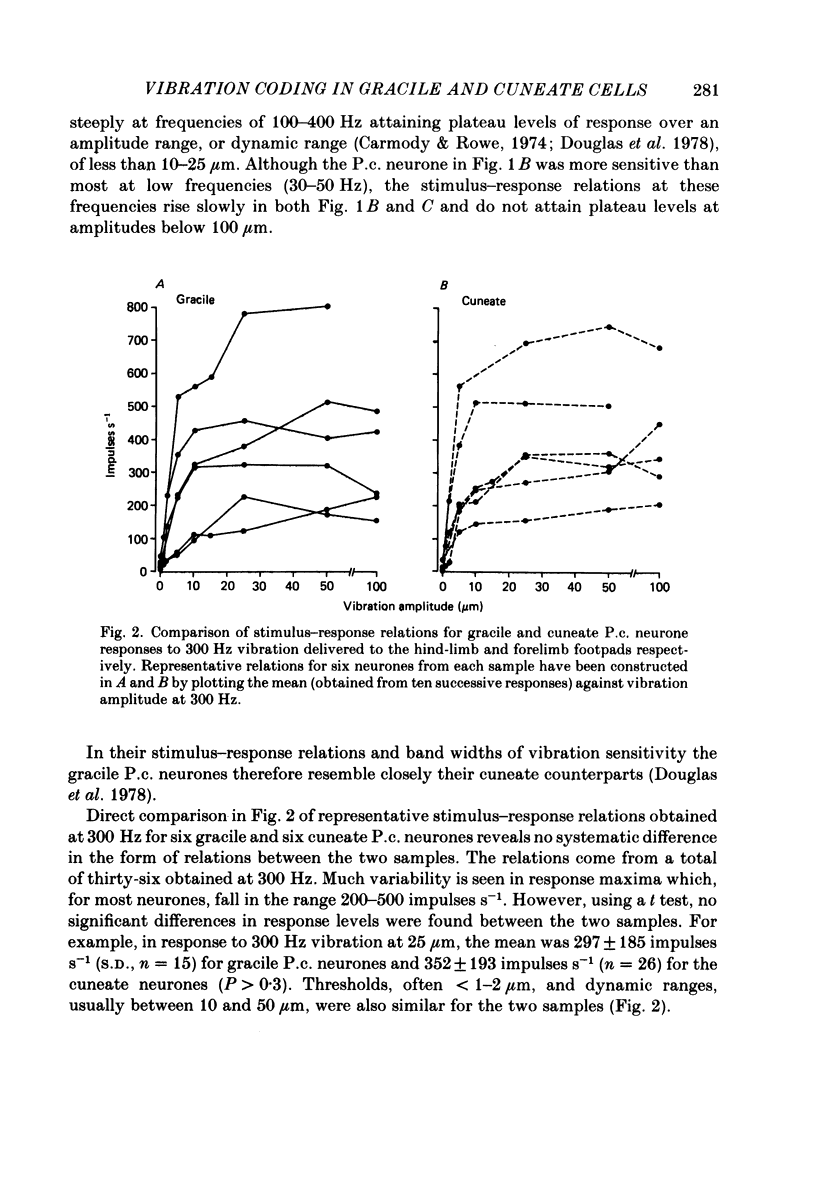
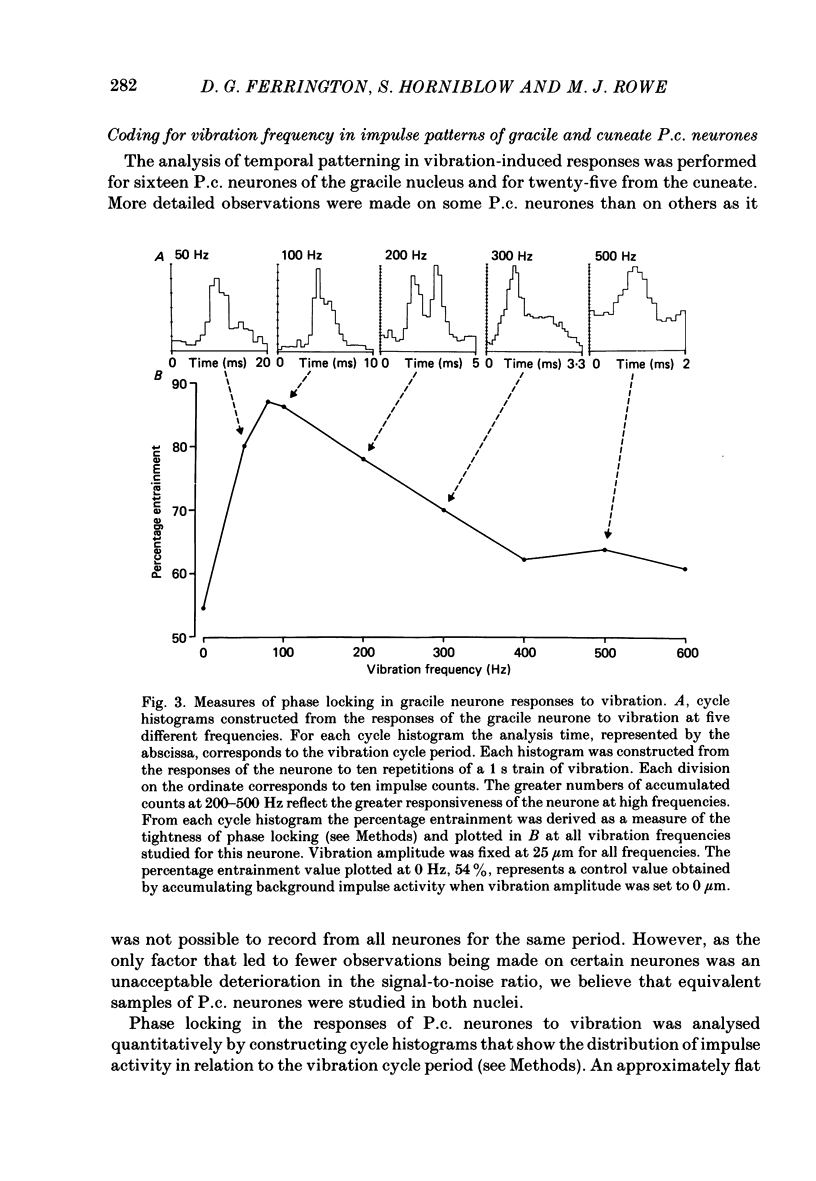
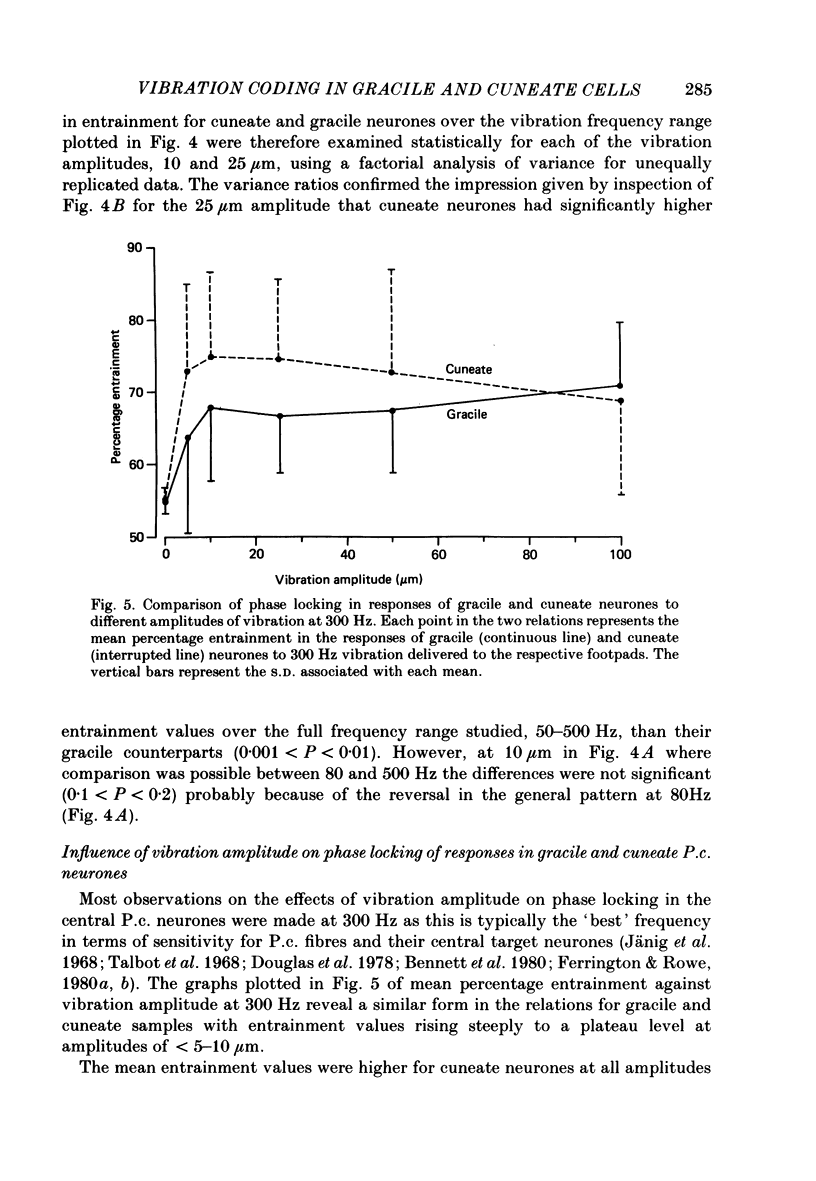
1. Recordings were made in decerebrate cats from gracile and cuneate neurones responding to vibration-induced inputs from Pacinian corpuscle (P.c.) receptors of the hind-limb and forelimb footpads. The two groups of neurones were compared, in particular for their capacities for responding to cutaneous vibration with phase-locked impulse patterns. 2. In both nuclei the P.c. neurones were most sensitive to vibration in the range 80 to greater than 600 Hz. Stimulus-response relations were similar for the two groups, as were measures derived from these relations such as response levels, absolute thresholds and the dynamic range (defined as the vibration amplitude range over which responses were graded). 3. At frequencies up to 300-400 Hz, responses for some neurones in both nuclei remained well phase locked to the vibration; however, quantitative analysis using a factorial analysis of variance indicated that the phase locking was poorer in gracile than cuneate neurones. 4. In both nuclei there was marked variability from neurone to neurone in measures of phase locking which may reflect variations in the extent of convergence of P.c. fibres upon different target neurones. For neurones in either nucleus that had comparatively tight phase locking of responses to vibration it is proposed that their output is functionally dominated by one or a few of their convergent P.c. input fibres.
Full text
PDF



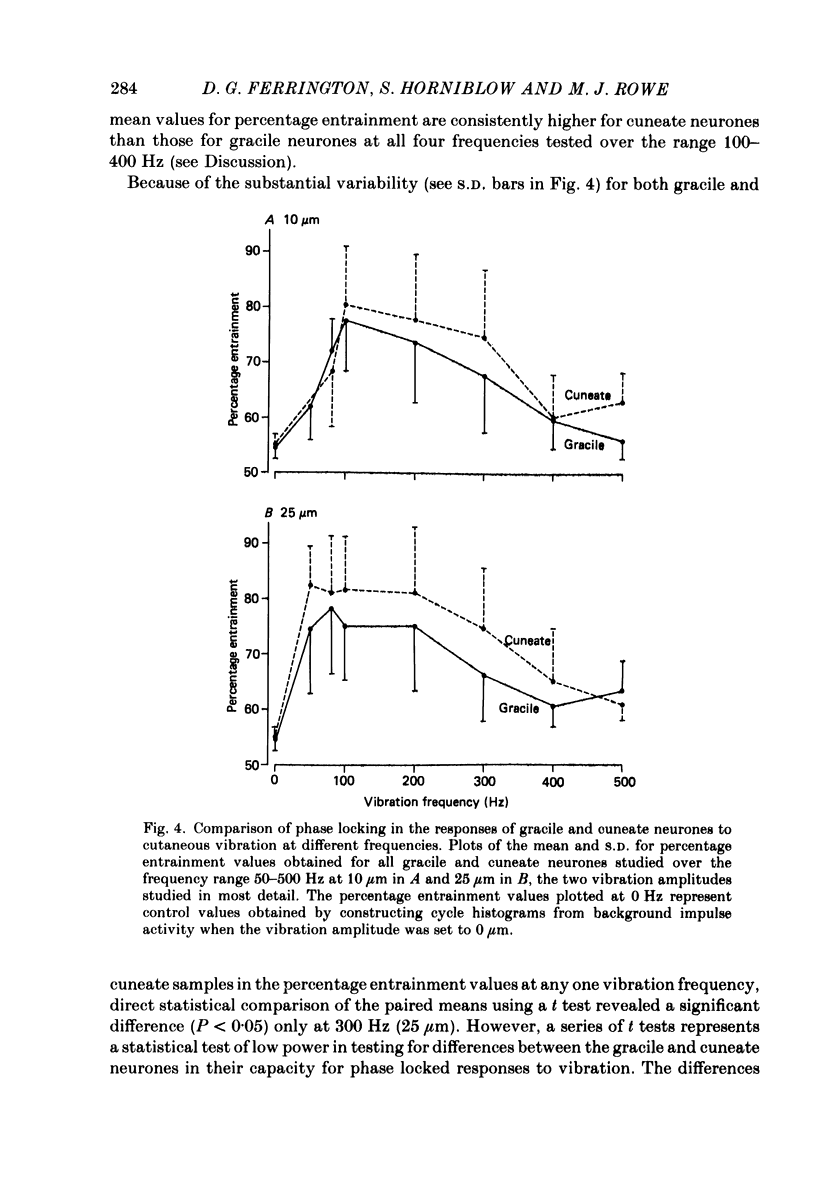

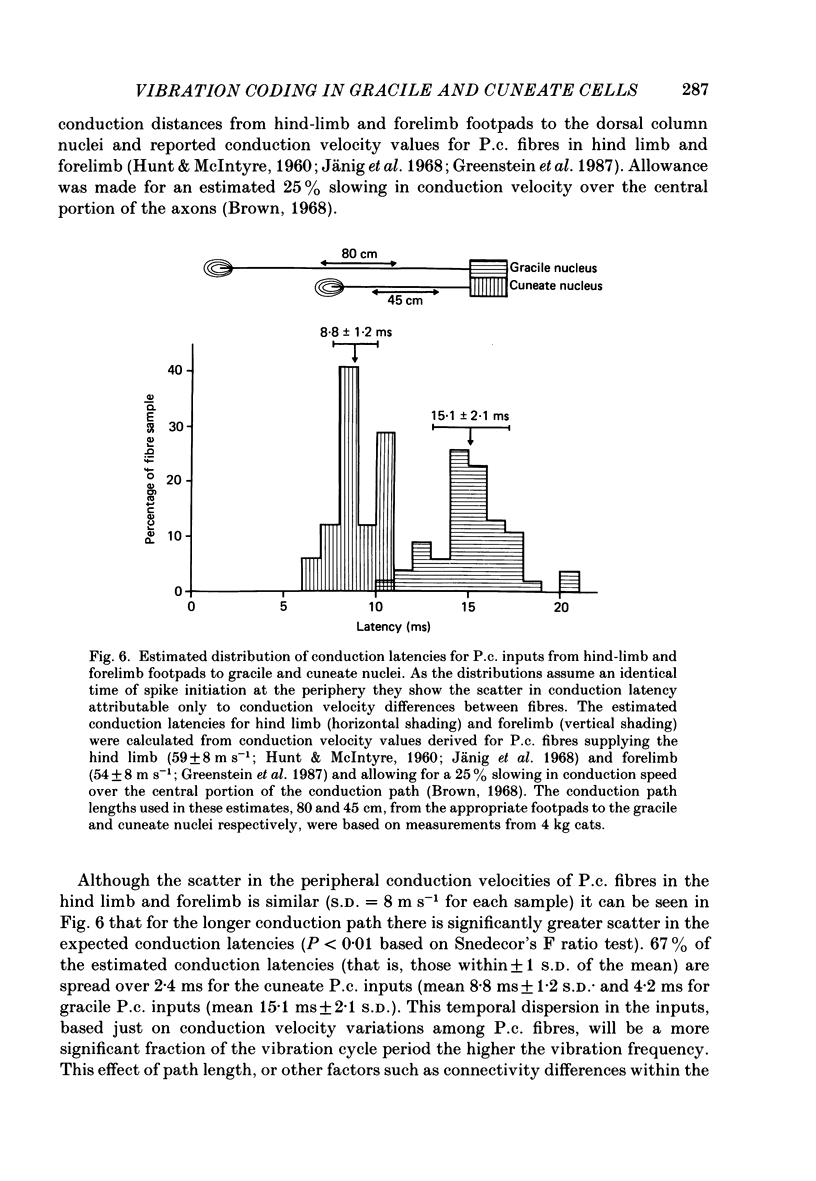




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. IDENTIFICATION OF RELAY CELLS AND INTERNEURONS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1080–1095. doi: 10.1152/jn.1964.27.6.1080. [DOI] [PubMed] [Google Scholar]
- Amassian V. E., Giblin D. Periodic components in steady-state activity of cuneate neurones and their possible role in sensory coding. J Physiol. 1974 Dec;243(2):353–385. doi: 10.1113/jphysiol.1974.sp010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. E., Ferrington D. G., Rowe M. Tactile neuron classes within second somatosensory area (SII) of cat cerebral cortex. J Neurophysiol. 1980 Feb;43(2):292–309. doi: 10.1152/jn.1980.43.2.292. [DOI] [PubMed] [Google Scholar]
- Berkley K. J. Different targets of different neurons in nucleus gracilis of the cat. J Comp Neurol. 1975 Oct 1;163(3):285–303. doi: 10.1002/cne.901630304. [DOI] [PubMed] [Google Scholar]
- Boivie J. The termination in the thalamus and the zona incerta of fibres from the dorsal column nuclei (DCN) in the cat. an experimental study with silver impregnation methods. Brain Res. 1971 May 21;28(3):459–490. doi: 10.1016/0006-8993(71)90056-4. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Cutaneous afferent fibre collaterals in the dorsal columns of the cat. Exp Brain Res. 1968;5(4):293–305. doi: 10.1007/BF00235904. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Gordon G., Kay R. H. A study of single axons in the cat's medial lemniscus. J Physiol. 1974 Jan;236(1):225–246. doi: 10.1113/jphysiol.1974.sp010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrzycka E., NAil B. S., Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive "tactile" neurones. J Physiol. 1977 Jun;268(1):251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J., Rowe M. Inhibition within the trigeminal nucleus induced by afferent inputs and its influence on stimulus coding by mechanosensitive neurones. J Physiol. 1974 Nov;243(1):195–210. doi: 10.1113/jphysiol.1974.sp010749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K. M., Ferrington D. G., Rowe M. J. Tactile sensory coding during development: signaling capacities of neurons in kitten dorsal column nuclei. J Neurophysiol. 1984 Jul;52(1):86–98. doi: 10.1152/jn.1984.52.1.86. [DOI] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G., RYAN R. D. FUNCTIONAL ORGANIZATION IN THE TRIGEMINAL MAIN SENSORY AND ROSTRAL SPINAL NUCLEI OF THE CAT. J Physiol. 1963 Aug;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P. R., Ferrington D. G., Rowe M. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978 Dec;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes R. W., Rasmusson D. D., Sretavan D., Rehman N. B. Submodality segregation and receptive-field sequences in cuneate, gracile, and external cuneate nuclei of the cat. J Neurophysiol. 1982 Mar;47(3):389–416. doi: 10.1152/jn.1982.47.3.389. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Hora M. O., Rowe M. J. Functional maturation of tactile sensory fibers in the kitten. J Neurophysiol. 1984 Jul;52(1):74–85. doi: 10.1152/jn.1984.52.1.74. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Functional capacities of tactile afferent fibres in neonatal kittens. J Physiol. 1980 Oct;307:335–353. doi: 10.1113/jphysiol.1980.sp013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J. Specificity of connections and tactile coding capacities in cuneate nucleus of the neonatal kitten. J Neurophysiol. 1982 Apr;47(4):622–640. doi: 10.1152/jn.1982.47.4.622. [DOI] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Actions of single sensory fibres on cat dorsal column nuclei neurones: vibratory signalling in a one-to-one linkage. J Physiol. 1987 May;386:293–309. doi: 10.1113/jphysiol.1987.sp016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. J., Tarvin R. P. Integrative processing of vibratory information in cat dorsal column nuclei neurones driven by identified sensory fibres. J Physiol. 1987 May;386:311–331. doi: 10.1113/jphysiol.1987.sp016536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington D. G., Rowe M. Differential contributions to coding of cutaneous vibratory information by cortical somatosensory areas I and II. J Neurophysiol. 1980 Feb;43(2):310–331. doi: 10.1152/jn.1980.43.2.310. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DUAL ORGANIZATION OF THE EXTEROCEPTIVE COMPONENTS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:263–290. doi: 10.1113/jphysiol.1964.sp007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., SEED W. A. An investigation of nucleus gracilis of the cat by antidromic stimulation. J Physiol. 1961 Mar;155:589–601. doi: 10.1113/jphysiol.1961.sp006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff G. D. Differential discrimination of frequency of cutaneous mechanical vibration. J Exp Psychol. 1967 Jun;74(2):294–299. doi: 10.1037/h0024561. [DOI] [PubMed] [Google Scholar]
- Greenstein J., Kavanagh P., Rowe M. J. Phase coherence in vibration-induced responses of tactile fibres associated with Pacinian corpuscle receptors in the cat. J Physiol. 1987 May;386:263–275. doi: 10.1113/jphysiol.1987.sp016533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Characteristics of responses from receptors from the flexor longus digitorum muscle and the adjoining interosseous region of the cat. J Physiol. 1960 Aug;153:74–87. doi: 10.1113/jphysiol.1960.sp006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. On the nature of vibration receptors in the hind limb of the cat. J Physiol. 1961 Jan;155:175–186. doi: 10.1113/jphysiol.1961.sp006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. On the use of nitrous oxide/oxygen mixtures for anaesthesia in cats [proceedings]. J Physiol. 1978 Feb;275:64P–64P. [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Lindblom U., Lund L. The discharge from vibration-sensitive receptors in the monkey foot. Exp Neurol. 1966 Aug;15(4):401–417. doi: 10.1016/0014-4886(66)90138-5. [DOI] [PubMed] [Google Scholar]
- Lynn B. The nature and location of certain phasic mechanoreceptors in the cat's foot. J Physiol. 1969 May;201(3):765–773. doi: 10.1113/jphysiol.1969.sp008786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. Loci of joint cells in the cuneate and external cuneate nuclei of the cat. Brain Res. 1979 May 11;167(2):385–390. doi: 10.1016/0006-8993(79)90832-1. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- PERL E. R., WHITLOCK D. G., GENTRY J. R. Cutaneous projection to second-order neurons of the dorsal column system. J Neurophysiol. 1962 May;25:337–358. doi: 10.1152/jn.1962.25.3.337. [DOI] [PubMed] [Google Scholar]
- Rothenberg M., Verrillo R. T., Zahorian S. A., Brachman M. L., Bolanowski S. J., Jr Vibrotactile frequency for encoding a speech parameter. J Acoust Soc Am. 1977 Oct;62(4):1003–1012. doi: 10.1121/1.381610. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Tracey D. J. The projection of joint receptors to the cuneate nucleus in the cat. J Physiol. 1980 Aug;305:433–449. doi: 10.1113/jphysiol.1980.sp013374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER D. L. N. GRACILIS OF CAT. FUNCTIONAL ORGANIZATION AND CORTICOFUGAL EFFECTS. J Neurophysiol. 1965 Jan;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]
- Willis W. D., Maunz R. A., Foreman R. D., Coulter J. D. Static and dynamic responses of spinothalamic tract neurons to mechanical stimuli. J Neurophysiol. 1975 May;38(3):587–600. doi: 10.1152/jn.1975.38.3.587. [DOI] [PubMed] [Google Scholar]


