Abstract
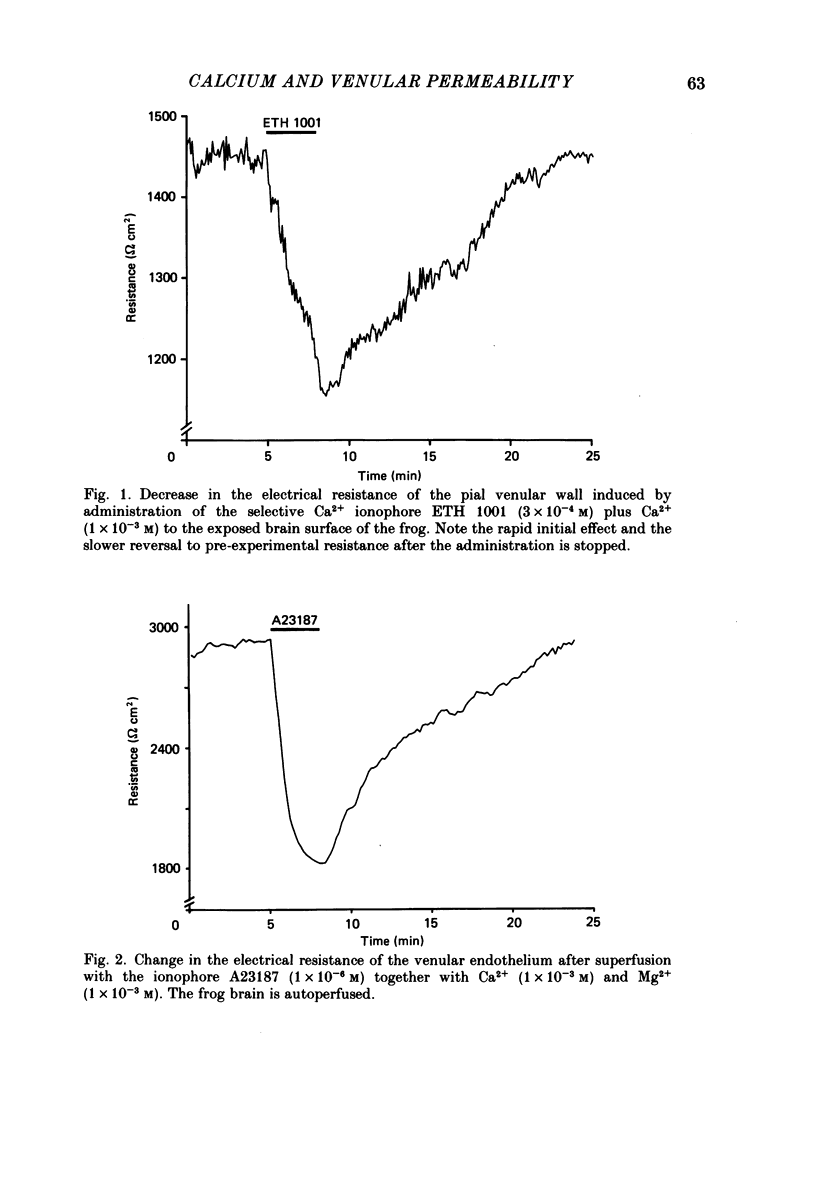
1. The effect on ionic permeability of frog brain endothelium of various second messengers was studied by a technique based on continuous recording of the electrical resistance of the venular endothelium in vivo. 2. Augmentation of the cytosolic Ca2+ concentration in endothelial cells induced with the ionophores ETH 1001 and A23187 increased ion permeability significantly as reflected in the reduced electrical resistance. 3. The electrical resistance fell reversibly within 1-2 s after administration of Ca2+-activating agents. The maximal effect was a reduction to about 0.70 times the pre-experimental resistance value. The resistance decrease was similar to that induced by several inflammatory mediators (Olesen & Crone, 1986). 4. Administration of the following agents did not change the electrical wall resistance: 8-bromo-cyclic AMP, dibutyryl-cyclic AMP, forskolin, 8-bromo-cyclic GMP, dibutyryl-cyclic GMP, sodium nitroprusside, phorbol myristate acetate (a protein kinase C stimulator). Changes in cytosolic Mg2+ did not affect permeability. 5. Ca2+ may be an important cytosolic signal in the endothelial cell, acting as an intracellular mediator for several permeability-augmenting substances.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Gamiño S. M., Rink T. J. Intracellular free magnesium in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1984 Sep;354:303–317. doi: 10.1113/jphysiol.1984.sp015377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Meza I., Martínez-Palomo A. Occluding junctions in cultured epithelial monolayers. Am J Physiol. 1981 Mar;240(3):C96–102. doi: 10.1152/ajpcell.1981.240.3.C96. [DOI] [PubMed] [Google Scholar]
- Crone C., Olesen S. P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982 Jun 3;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Role of calcium and phosphoinositides in the actions of certain hormones and neurotransmitters. J Clin Invest. 1985 Jun;75(6):1753–1757. doi: 10.1172/JCI111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P., Lew V. L. Use of ionophore A23187 to measure and to control free and bound cytoplasmic Mg in intact red cells. Nature. 1977 May 26;267(5609):360–362. doi: 10.1038/267360a0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Grega G. J., Maciejko J. J., Raymond R. M., Sak D. P. The interrelationship among histamine, various vasoactive substances, and macromolecular permeability in the canine forelimb. Circ Res. 1980 Feb;46(2):264–275. doi: 10.1161/01.res.46.2.264. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Hinds T. R., Vincenzi F. F. The effect of ETH 1001 on ion fluxes across red blood cell membranes. Cell Calcium. 1985 Jun;6(3):265–279. doi: 10.1016/0143-4160(85)90011-9. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., Michel C. C. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Joyner W. L. The effect of altering the external calcium concentration and a calcium channel blocker, verapamil, on microvascular leaky sites and dextran clearance in the hamster cheek pouch. Microvasc Res. 1984 Sep;28(2):159–179. doi: 10.1016/0026-2862(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Michel C. C. The Malpighi lecture. Vascular permeability--the consequence of Malpighi's hypothesis. Int J Microcirc Clin Exp. 1985;4(3):265–284. [PubMed] [Google Scholar]
- Nicolaysen G. Increase in capillary filtration rate resulting from reduction in the intravascular calcium ion-concentration. Acta Physiol Scand. 1971 Apr;81(4):517–527. doi: 10.1111/j.1748-1716.1971.tb04929.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. A calcium-dependent reversible permeability increase in microvessels in frog brain, induced by serotonin. J Physiol. 1985 Apr;361:103–113. doi: 10.1113/jphysiol.1985.sp015635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P., Crone C. Substances that rapidly augment ionic conductance of endothelium in cerebral venules. Acta Physiol Scand. 1986 Jun;127(2):233–241. doi: 10.1111/j.1748-1716.1986.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., de Saint-Aubain M. L., Bundgaard M. Permeabilities of single arterioles and venules in the frog skin: a functional and morphological study. Microvasc Res. 1984 Jul;28(1):1–22. doi: 10.1016/0026-2862(84)90025-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H., Yin H. L., Southwick F. S., Zaner K. S. The motor of leukocytes. Fed Proc. 1984 Sep;43(12):2760–2763. [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Vestergaard-Bogind B., Stampe P. Trans to cis proton concentration gradients accelerate ionophore A23187-mediated net fluxes of Ca2+ across the human red cell membrane. Biochim Biophys Acta. 1984 Sep 5;775(3):328–340. doi: 10.1016/0005-2736(84)90188-3. [DOI] [PubMed] [Google Scholar]
- Weeds A. Actin-binding proteins--regulators of cell architecture and motility. Nature. 1982 Apr 29;296(5860):811–816. doi: 10.1038/296811a0. [DOI] [PubMed] [Google Scholar]


