Abstract
1. The gating property of a mechano-electrical transduction (m-e.t.) was studied in dissociated chick vestibular hair cells having hair bundles of varying length (from 7 to more than 30 microns long). The whole-cell recording voltage-clamp technique was used to record m-e.t. currents, and mechanical stimuli were applied to the hair bundle with a rigid glass rod. 2. Displacements of the glass rod and the hair bundle were measured with a resolution of 0.1 micron from contrast-enhanced television images. The motion of the hair bundle was tightly coupled to the motion of the stimulating glass rod, and displacements were not detected in the circumference of the cell body. Thus, all the displacement applied to the hair bundle resulted in bending about the insertion into the cuticle and relative to the cell body. 3. Displacements of the hair bundle towards the taller stereocilia generated inward-going m-e.t. currents at negative membrane potentials, while displacements of the hair bundle towards the shorter stereocilia generated outward-going m-e.t. currents. These outward going m-e.t. currents reflect closing of the m-e.t. channels which are open at the resting position of the hair bundle. The fraction of these channels open at the resting position was 0.12 +/- 0.04 (n = 7). 4. The displacement-response relationship measured both at -50 mV and at +38 mV were superimposable after scaling. Thus, no voltage dependence was observed in gating of the m-e.t. conductance. 5. When a hair bundle of a shorter length (less than 7.5 microns long) was stimulated at 5 micron form the insertion to the cuticle, the minimum hair-bundle displacement which could generate a detectable amount of m-e.t. current was 0.01 micron. The transduction current was linearly related to the hair-bundle displacement for values of up to 0.6 micron towards the taller stereocilia, and showed saturation with larger displacements. 6. When a hair bundle of a longer length (more than 12.5 microns long) was stimulated (towards the taller stereocilia) at 10 micron from the insertion to the cuticle the m-e.t. current generated was linear for displacements of up to 1.5 micron, and saturated with larger displacements. 7. The above two points suggest that the range of linear transduction becomes wider as the length of the hair bundle becomes longer under in situ conditions where the displacement is likely to be applied at the tip of the hair bundle.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

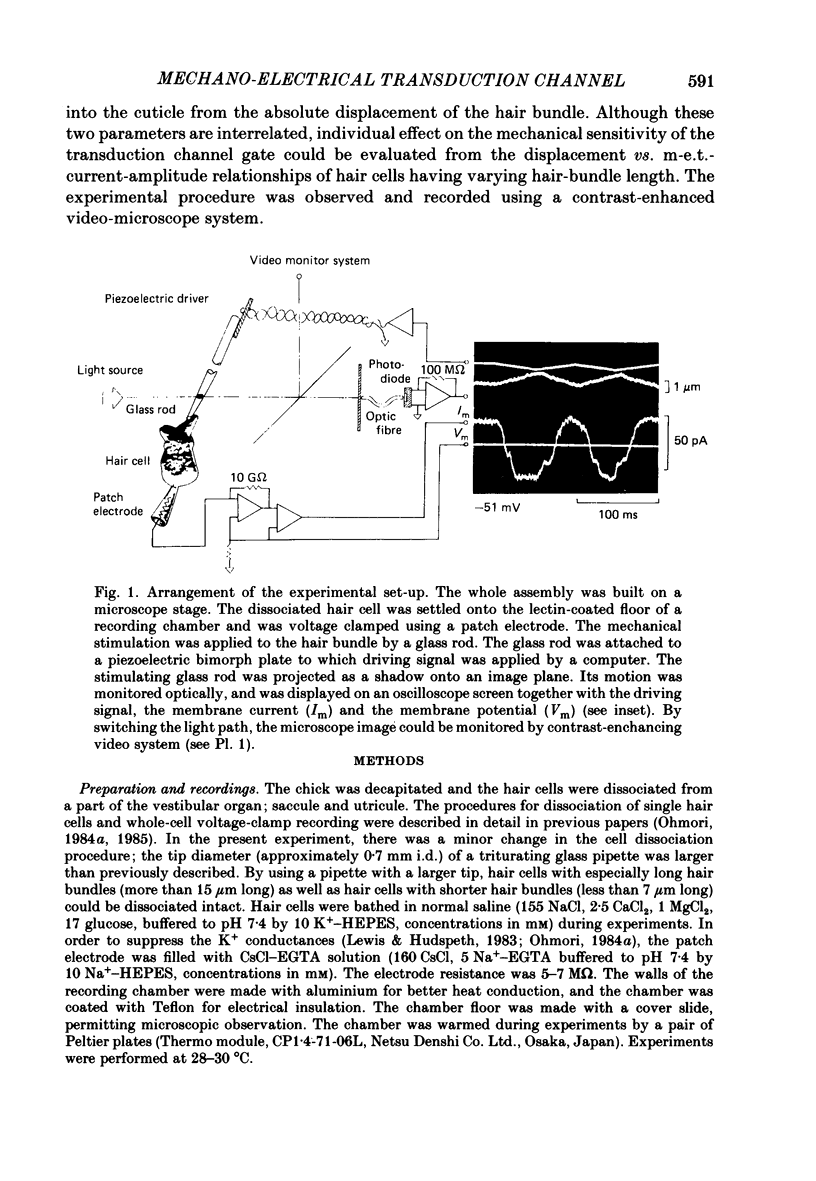

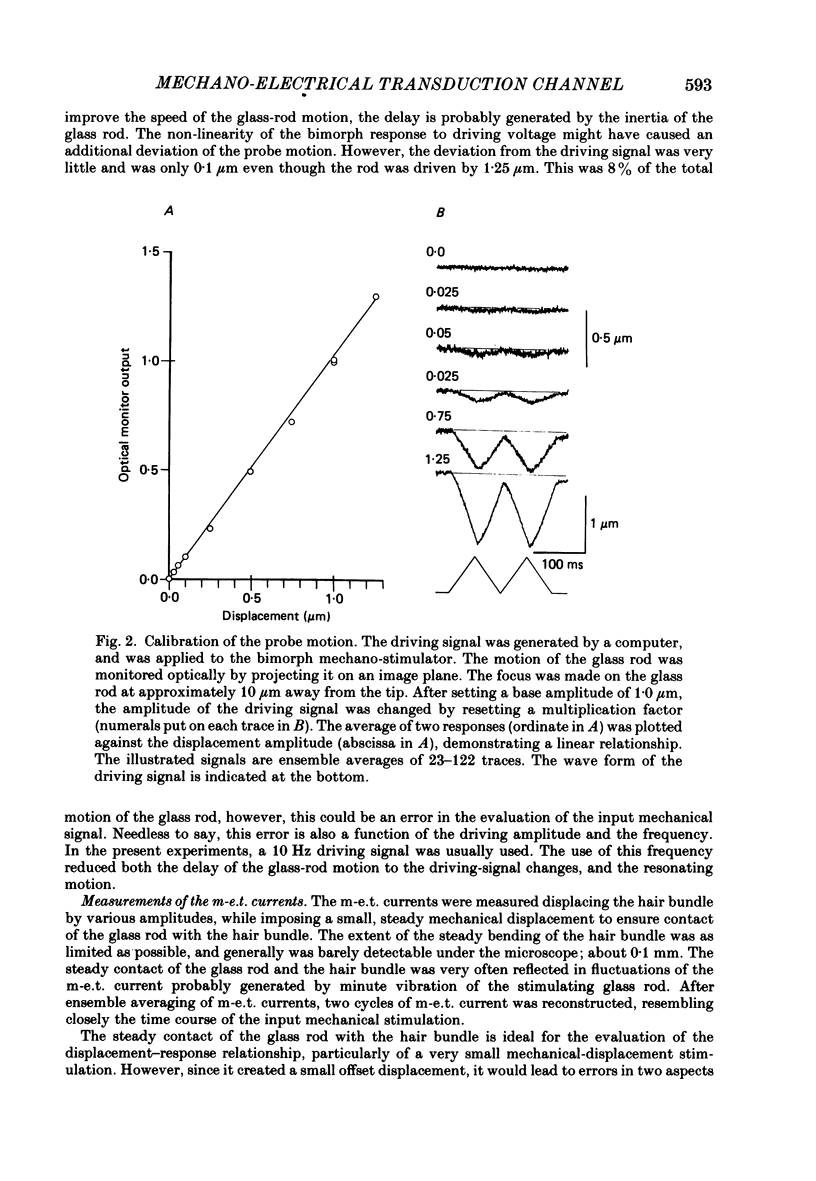
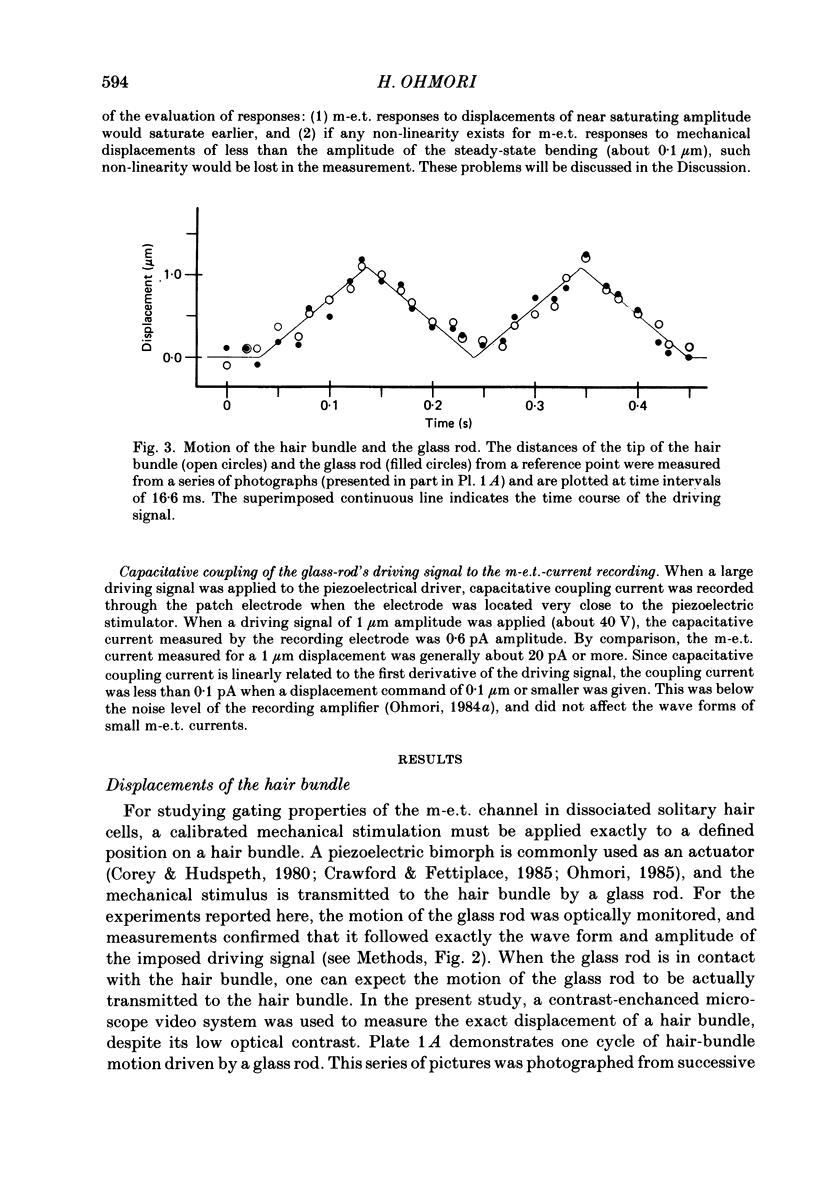

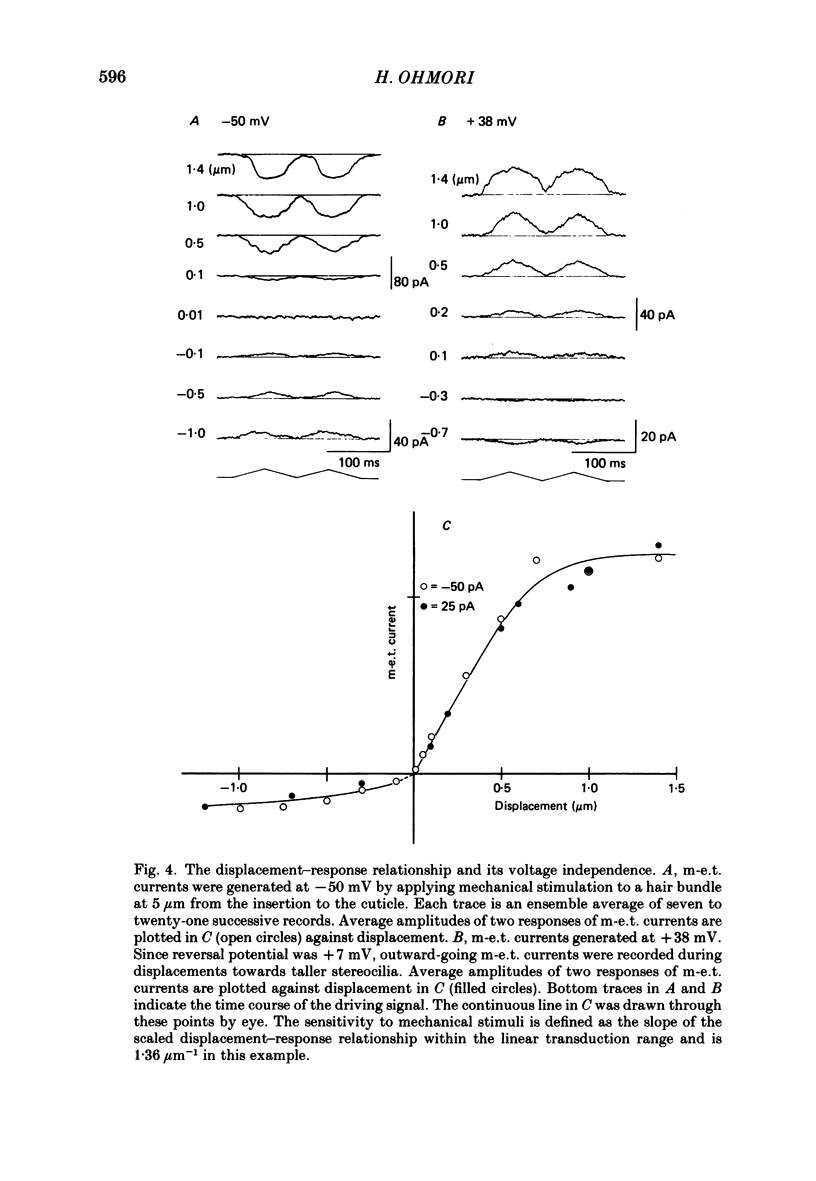


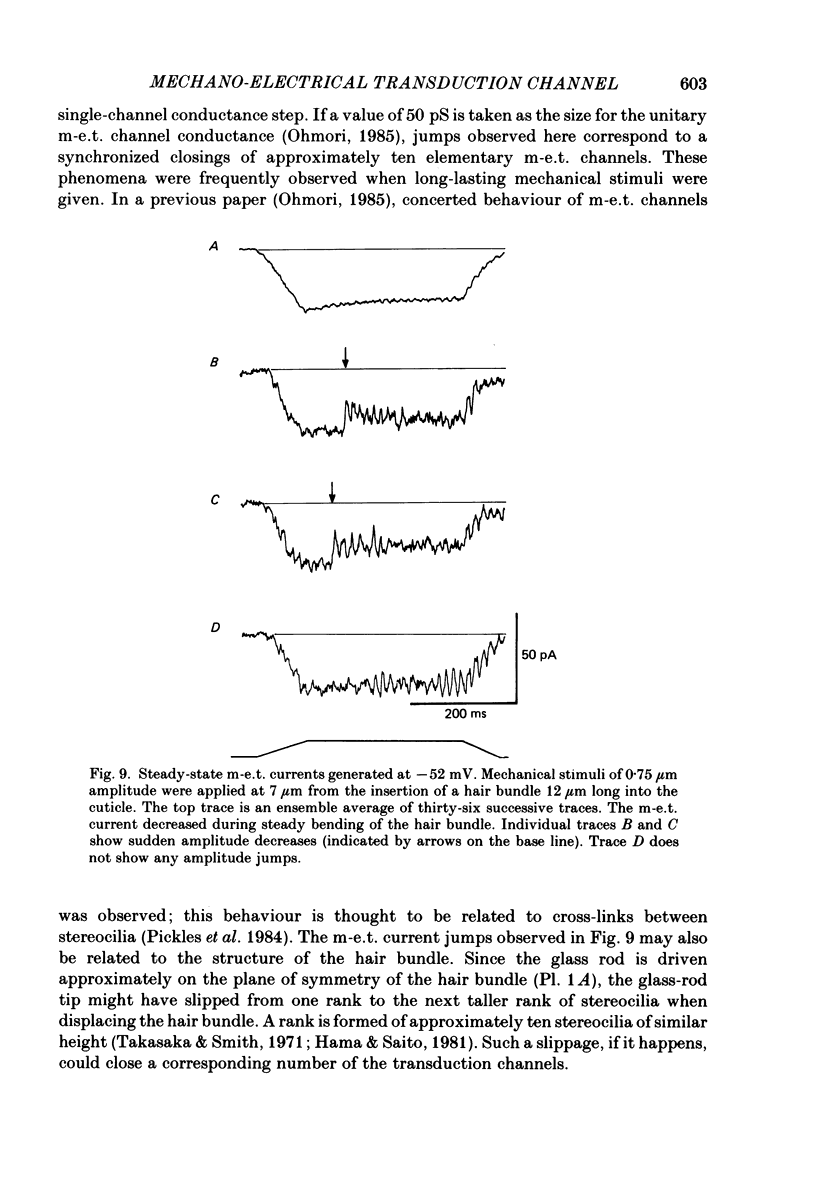






Images in this article
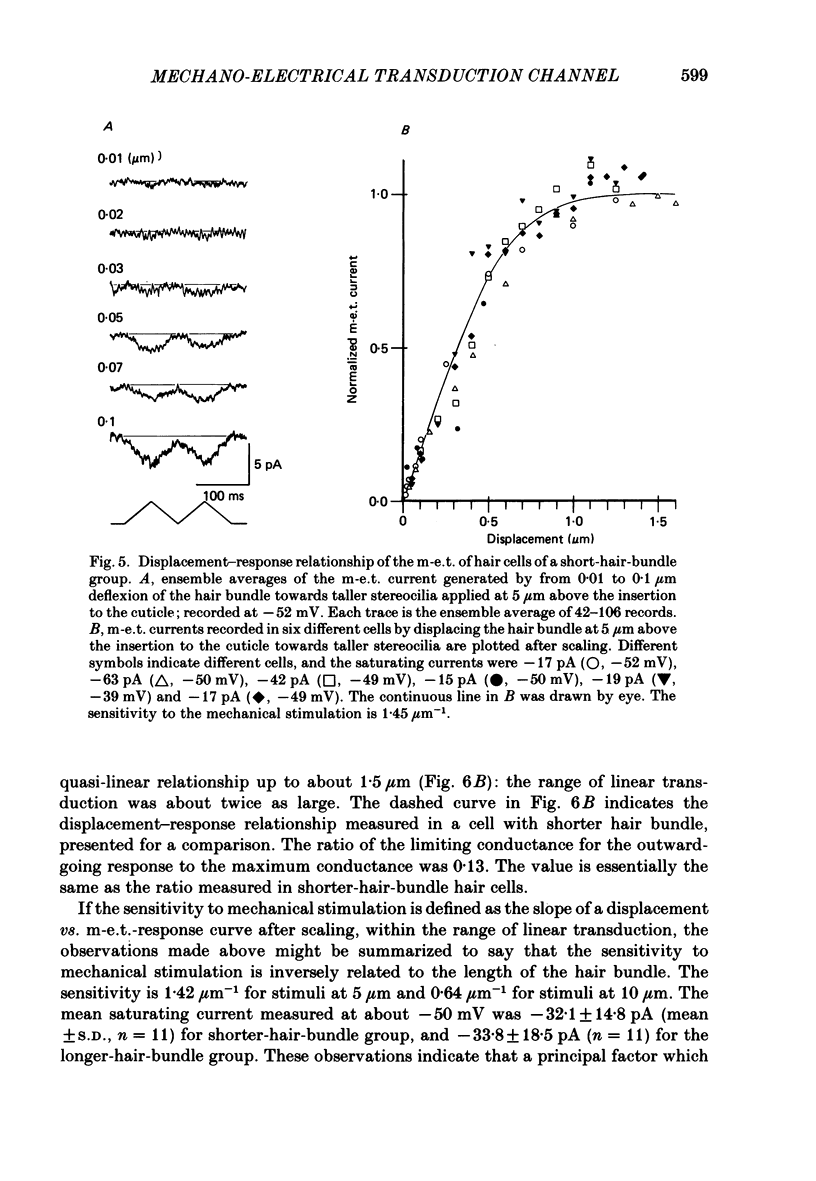
Selected References
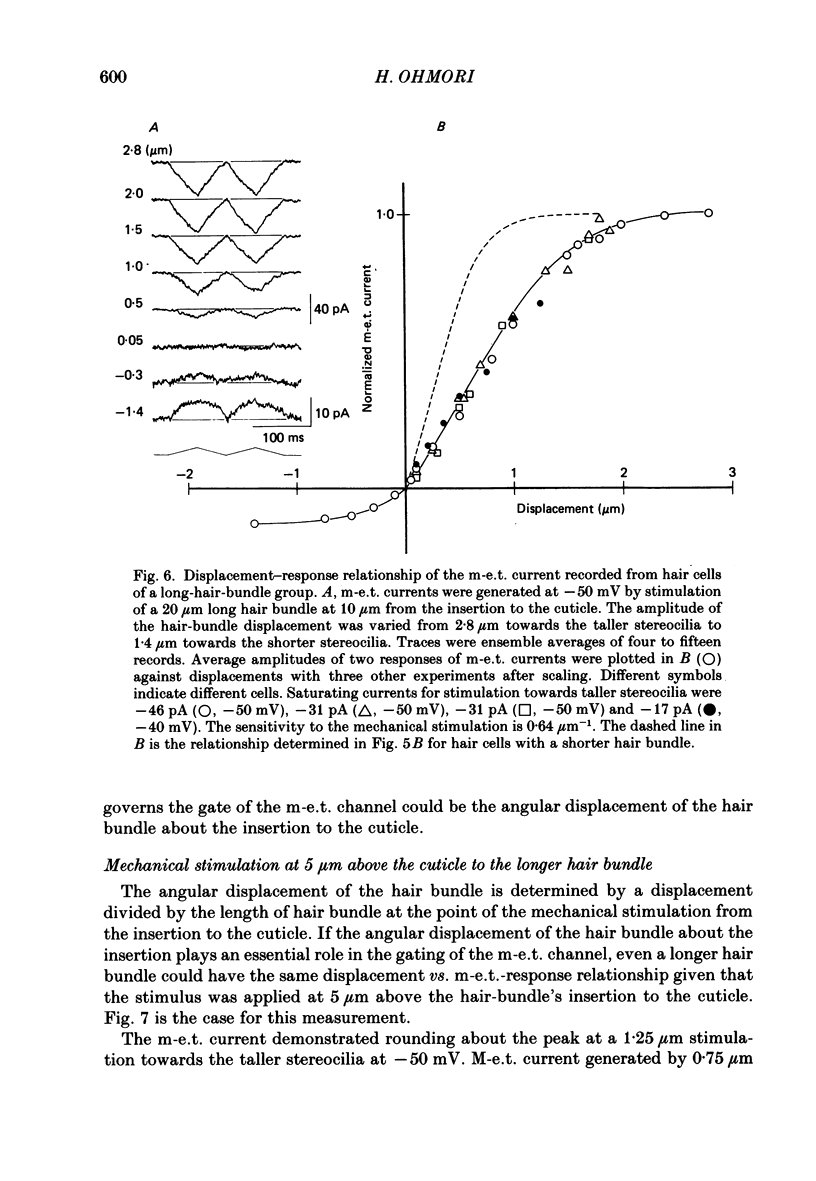
These references are in PubMed. This may not be the complete list of references from this article.
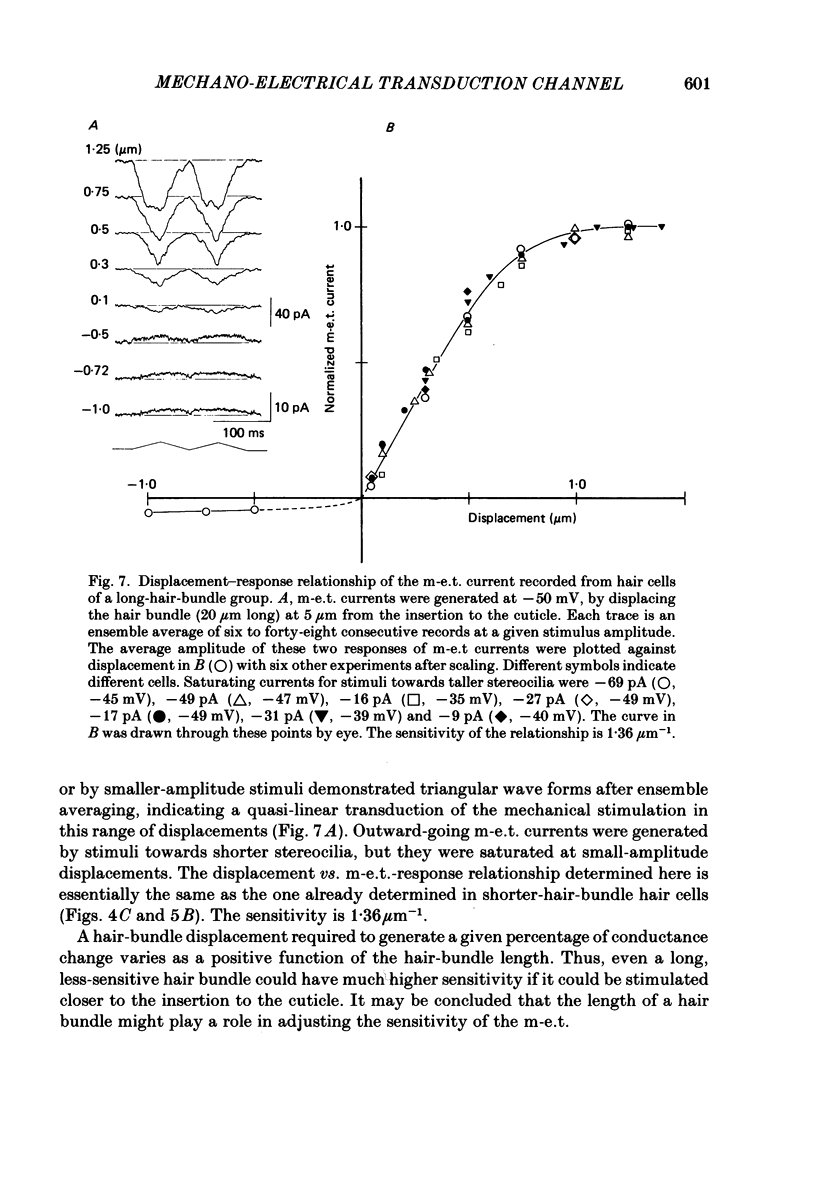
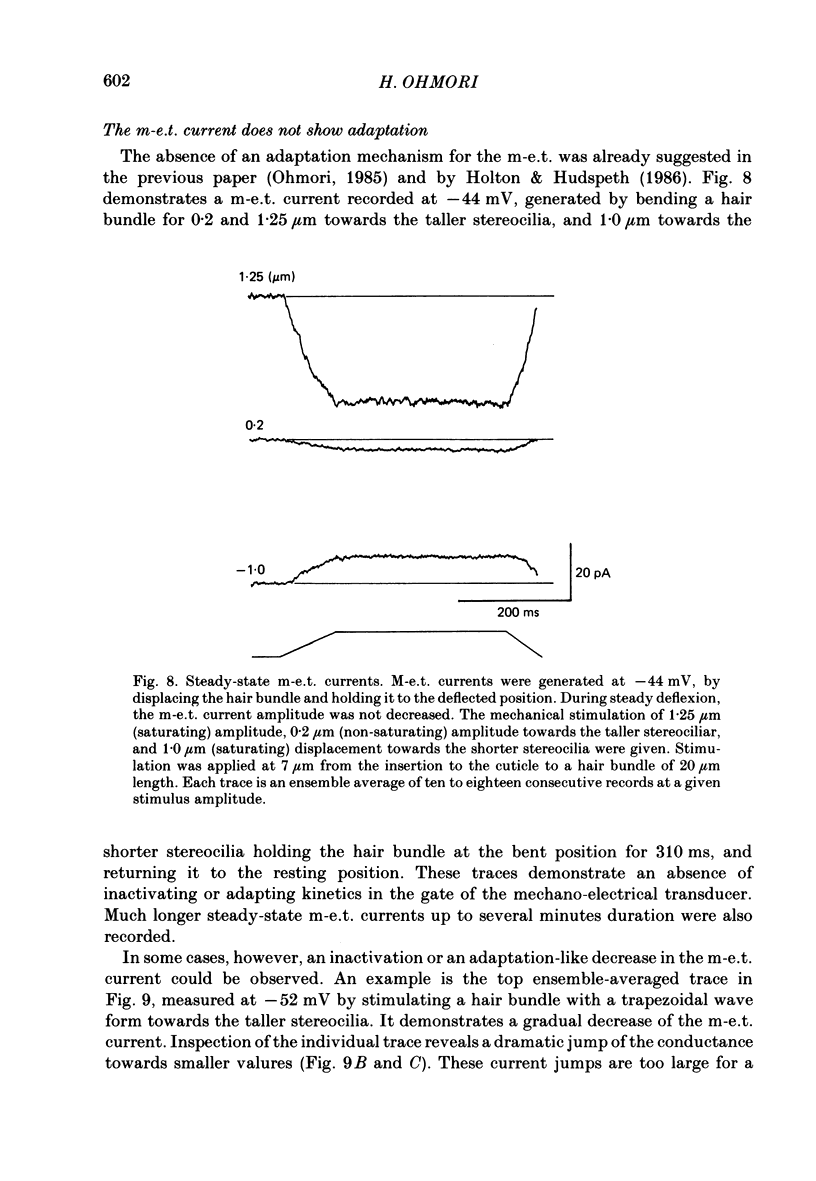
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978 Jun 1;273(5661):377–378. doi: 10.1038/273377a0. [DOI] [PubMed] [Google Scholar]
- CITRON L., EXLEY D., HALLPIKE C. S. Formation, circulation and chemical properties of the labyrinthine fluids. Br Med Bull. 1956 May;12(2):101–104. doi: 10.1093/oxfordjournals.bmb.a069529. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Analysis of the microphonic potential of the bullfrog's sacculus. J Neurosci. 1983 May;3(5):942–961. doi: 10.1523/JNEUROSCI.03-05-00942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Mechanical stimulation and micromanipulation with piezoelectric bimorph elements. J Neurosci Methods. 1980 Dec;3(2):183–202. doi: 10.1016/0165-0270(80)90025-4. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985 Jul;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci. 1985 Jun;5(6):1591–1608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. A model for transducer action in the cochlea. Cold Spring Harb Symp Quant Biol. 1965;30:181–190. doi: 10.1101/sqb.1965.030.01.020. [DOI] [PubMed] [Google Scholar]
- Engström H., Engström B. Structure of the hairs on cochlear sensory cells. Hear Res. 1978 Oct;1(1):49–66. doi: 10.1016/0378-5955(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Flock A., Flock B., Murray E. Studies on the sensory hairs of receptor cells in the inner ear. Acta Otolaryngol. 1977 Jan-Feb;83(1-2):85–91. doi: 10.3109/00016487709128817. [DOI] [PubMed] [Google Scholar]
- Hillman D. E., Lewis E. R. Morphological basis for a mechanical linkage in otolithic receptor transduction in the frog. Science. 1971 Oct 22;174(4007):416–419. doi: 10.1126/science.174.4007.416. [DOI] [PubMed] [Google Scholar]
- Holton T., Hudspeth A. J. The transduction channel of hair cells from the bull-frog characterized by noise analysis. J Physiol. 1986 Jun;375:195–227. doi: 10.1113/jphysiol.1986.sp016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Corey D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechanoelectrical transducer has discrete conductances in the chick vestibular hair cell. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1888–1891. doi: 10.1073/pnas.81.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984 Aug;15(2):103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Russell I. J. Origin of the receptor potential in inner hair cells of the mammalian cochlea--evidence for Davis' theory. Nature. 1983 Jan 27;301(5898):334–336. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Richardson G. P., Cody A. R. Mechanosensitivity of mammalian auditory hair cells in vitro. 1986 May 29-Jun 4Nature. 321(6069):517–519. doi: 10.1038/321517a0. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick P. M., Johnstone B. M. Production and role of inner ear fluid. Prog Neurobiol. 1975;5(4):337–362. doi: 10.1016/0301-0082(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Strelioff D., Flock A. Stiffness of sensory-cell hair bundles in the isolated guinea pig cochlea. Hear Res. 1984 Jul;15(1):19–28. doi: 10.1016/0378-5955(84)90221-1. [DOI] [PubMed] [Google Scholar]
- Takasaka T., Smith C. A. The structure and innervation of the pigeon's basilar papilla. J Ultrastruct Res. 1971 Apr;35(1):20–65. doi: 10.1016/s0022-5320(71)80141-7. [DOI] [PubMed] [Google Scholar]
- Turner R. G., Muraski A. A., Nielsen D. W. Cilium length: influence on neural tonotopic organization. Science. 1981 Sep 25;213(4515):1519–1521. doi: 10.1126/science.7280673. [DOI] [PubMed] [Google Scholar]



