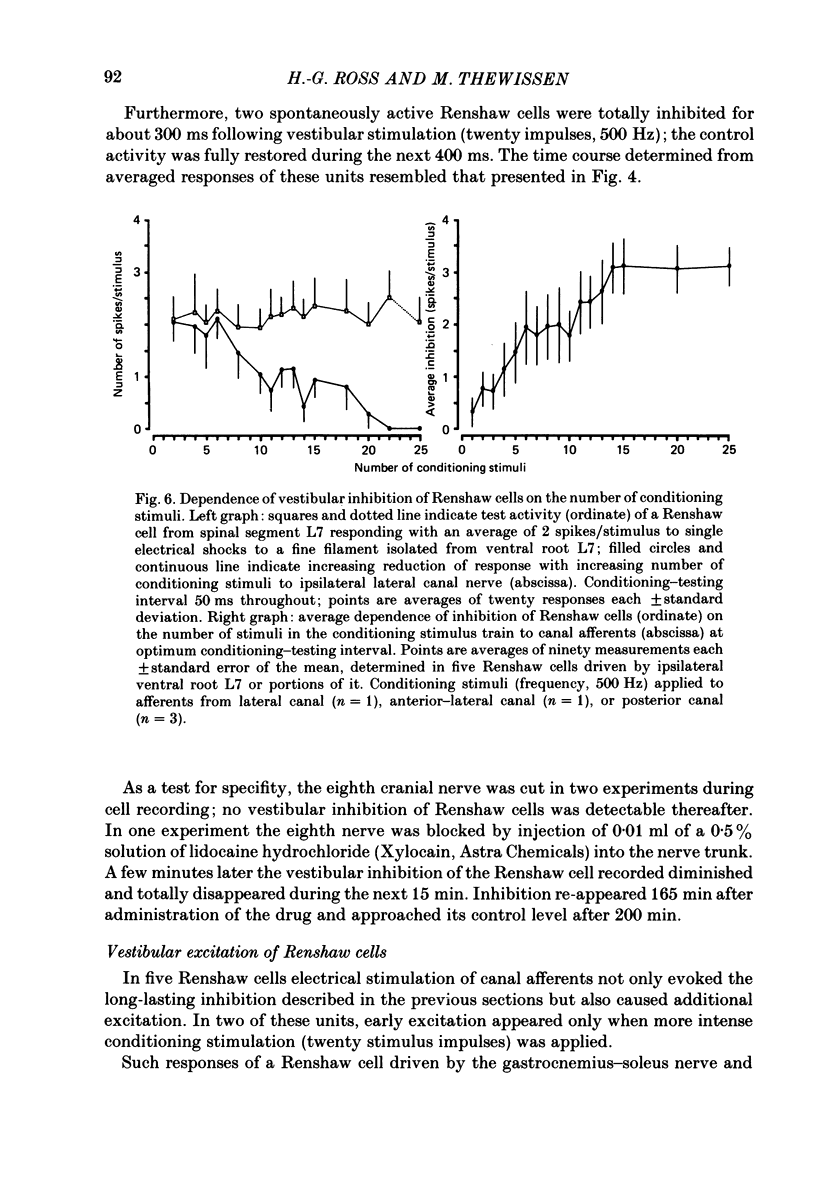
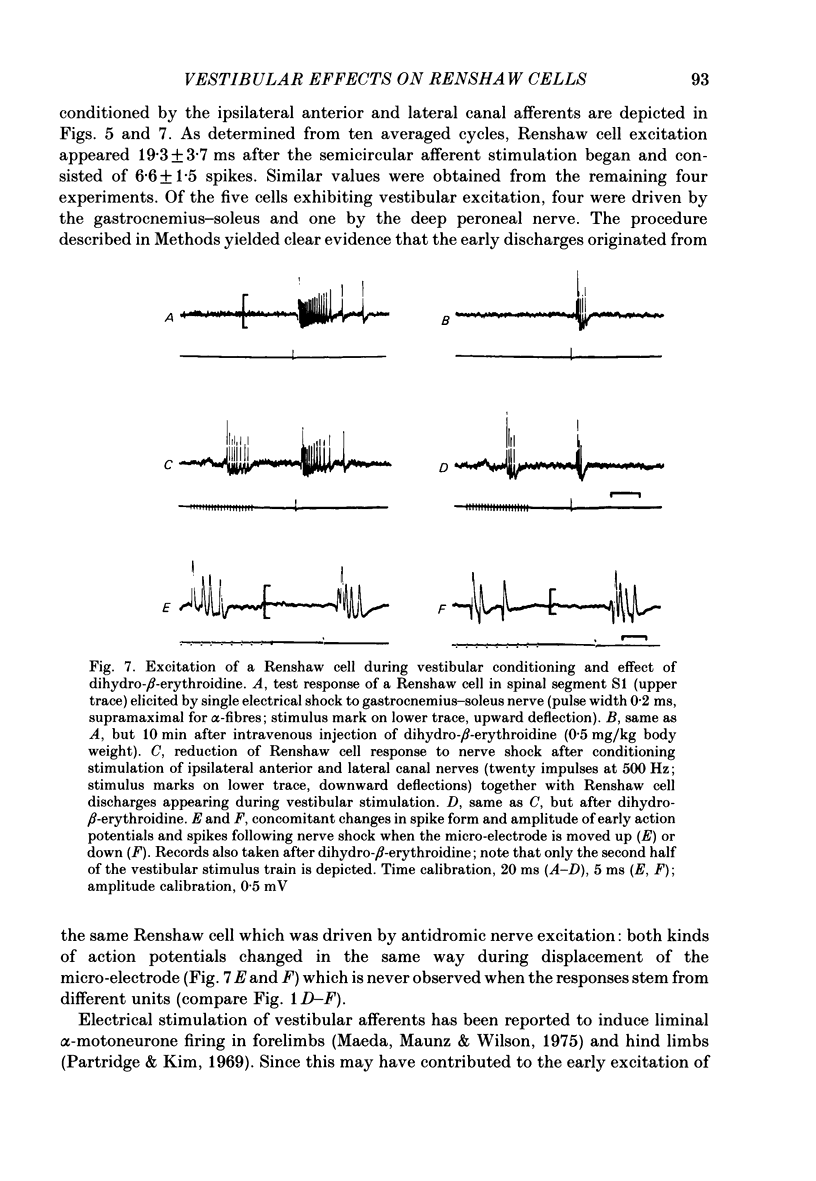
Abstract
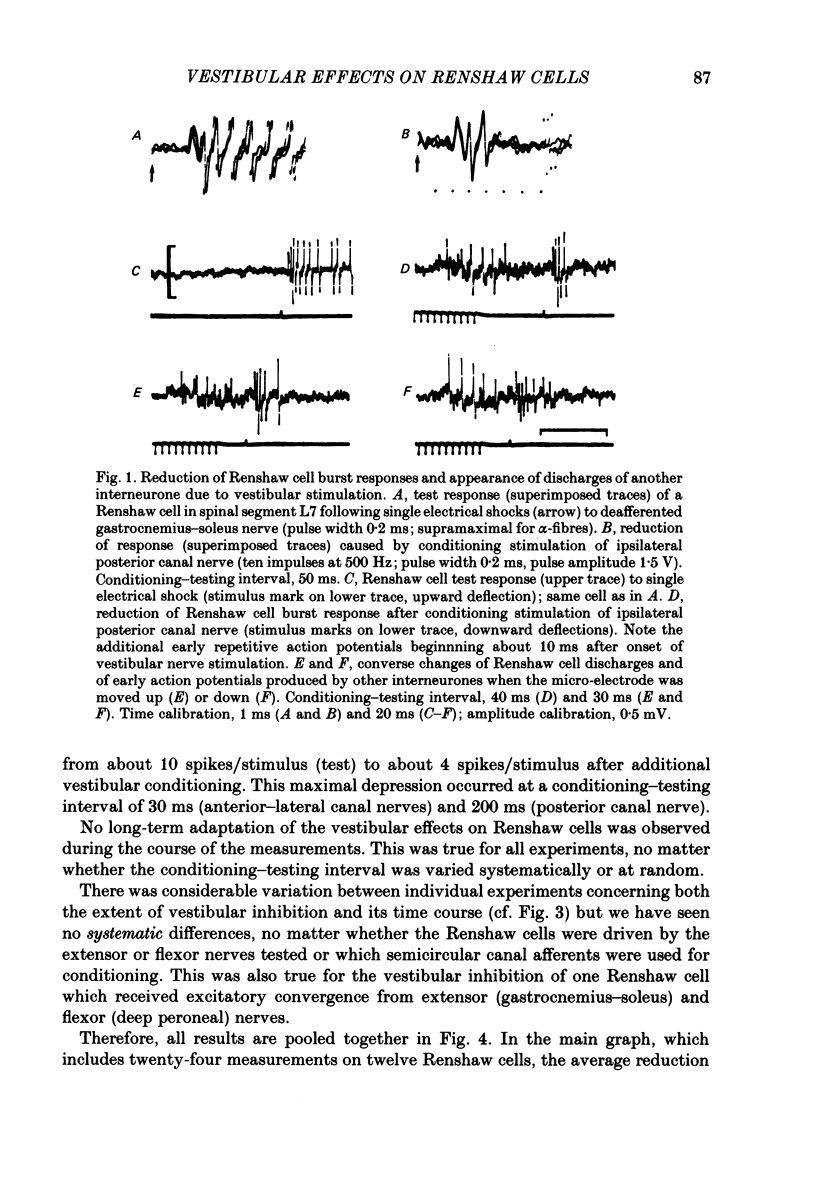
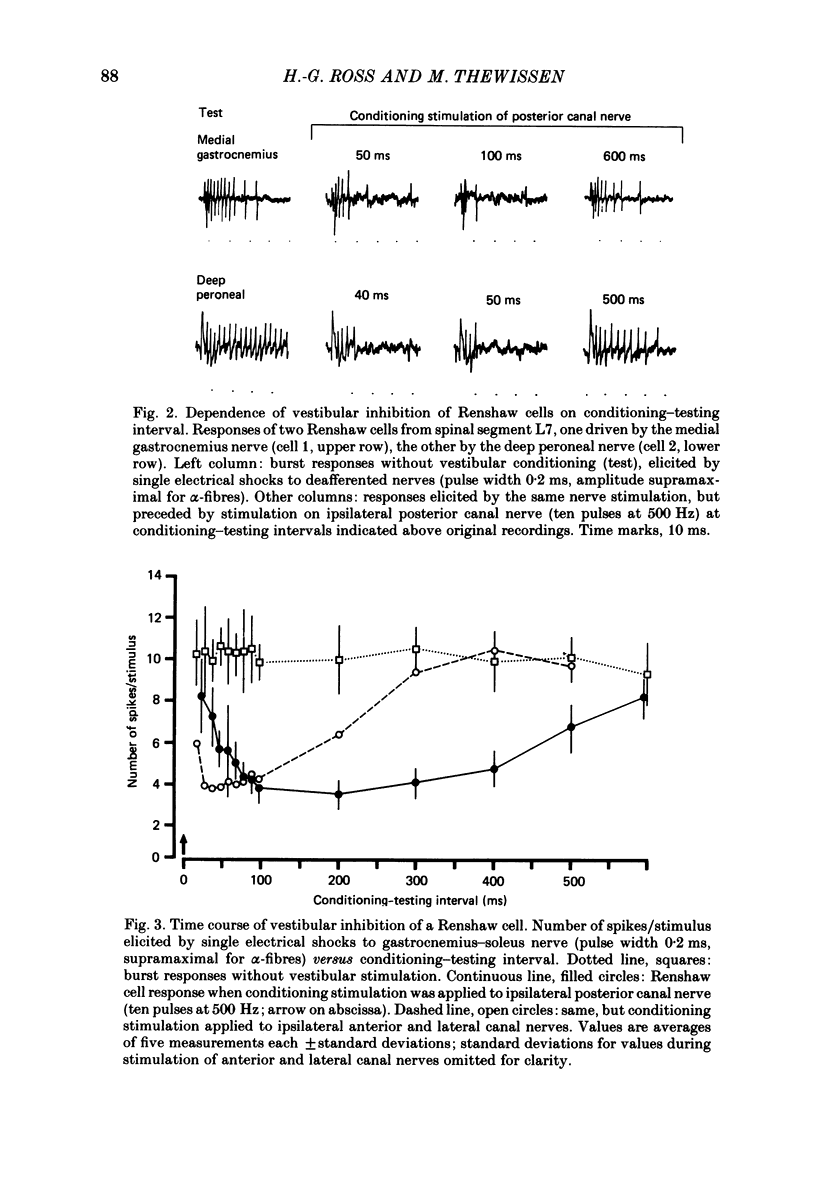
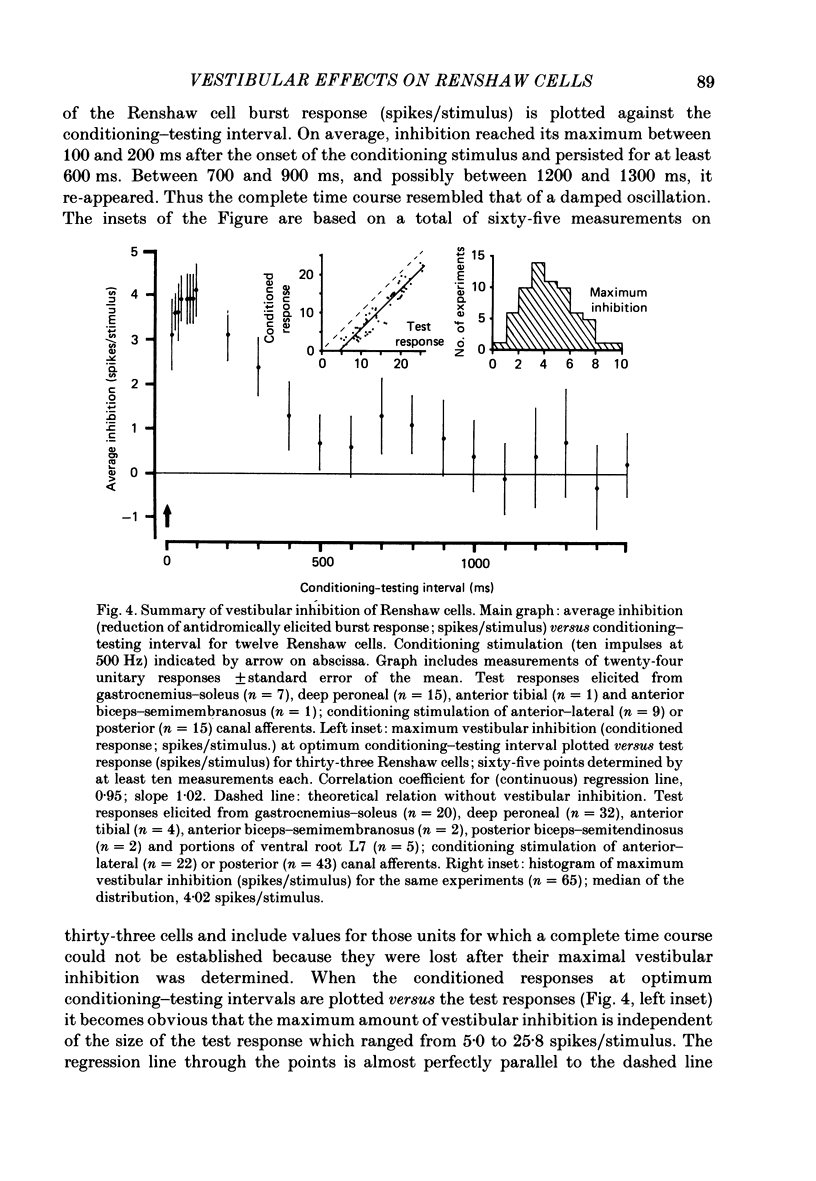
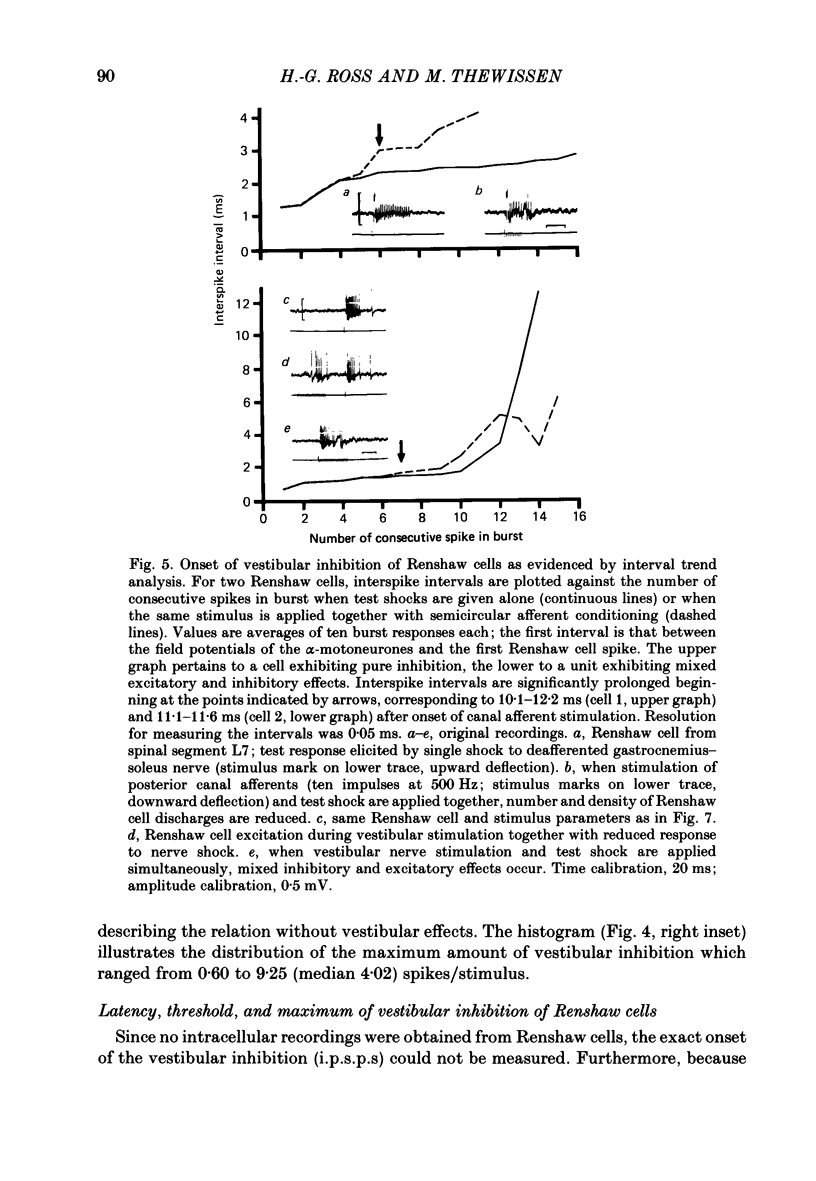
1. In intercollicularly decerebrate cats, the excitability of lumbar spinal Renshaw cells (tested by single shocks to ventral roots and deafferented muscle nerves) decreased for 600-1000 ms after conditioning electrical stimulation of ipsilateral semicircular canal nerves. 2. Conditioning stimulation of posterior canal afferents and combined stimulation of anterior and lateral canal afferents were equally effective in causing inhibition of Renshaw cells. No significant differences were observed for Renshaw cells excitable from hind-limb flexor or extensor nerves. 3. Inhibition appeared when one to five stimuli were applied to the canal afferents and arrived at the spinal segmental level 11-15 ms after the onset of conditioning stimulation. 4. Evidence is adduced to suggest that the inhibitory effects on Renshaw cells following stimulation of semicircular canal afferents were mediated directly, i.e. they were not caused by alterations of motoneurone activity. 5. Excitation of Renshaw cells due to stimulation of the canal afferents was rarely observed; it could not be excluded that it was secondary to motoneurone discharges. 6. It is suggested that vestibular inhibition of Renshaw cells ensure a high gain of hind-limb alpha-motoneurones during postural adjustments following a massive disturbance of body equilibrium.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Cleveland S., Haase J., Ross H. G., Wand P. Antidromic conditioning of reciprocally inhibited monosynaptic extensor and flexor reflexes in decerebrate cats. Pflugers Arch. 1972;337(3):219–228. doi: 10.1007/BF00586846. [DOI] [PubMed] [Google Scholar]
- Cleveland S., Kuschmierz A., Ross H. G. Static input-output relations in the spinal recurrent inhibitory pathway. Biol Cybern. 1981;40(3):223–231. doi: 10.1007/BF00453372. [DOI] [PubMed] [Google Scholar]
- Cook W. A., Jr, Cangiano A., Pompeiano O. Vestibular control of transmission in primary afferents to the lumbar spinal cord. Arch Ital Biol. 1969 Aug;107(3):296–320. [PubMed] [Google Scholar]
- Deliagina T. G., Feldman A. G. Activity of Renshaw cells during fictive scratch reflex in the cat. Exp Brain Res. 1981;42(1):108–115. doi: 10.1007/BF00235735. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological investigations on Renshaw cells. J Physiol. 1961 Dec;159:461–478. doi: 10.1113/jphysiol.1961.sp006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Unitary activity of spinal interneurones of cats. J Physiol. 1956 Feb 28;131(2):424–435. doi: 10.1113/jphysiol.1956.sp005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Cleveland S., Ross H. G. Problems of postsynaptic autogenous and recurrent inhibition in the mammalian spinal cord. Rev Physiol Biochem Pharmacol. 1975;73:73–129. doi: 10.1007/BFb0034660. [DOI] [PubMed] [Google Scholar]
- Haase J., Vogel B. Die Erregung der Renshaw-Zellen durch reflektorische Entladungen der alpha-Motoneurone. Pflugers Arch. 1971;325(1):14–27. doi: 10.1007/BF00587488. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Hultborn H., Jespersen B., Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55(2):391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Lindström S., Wigström H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979 Oct;37(2):399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979 Dec;297(0):229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Input-output relations in the pathway of recurrent inhibition to motoneurones in the cat. J Physiol. 1979 Dec;297(0):267–287. doi: 10.1113/jphysiol.1979.sp013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R., Pierrot-Deseilligny E. Facilitation of soleus-coupled Renshaw cells during voluntary contraction of pretibial flexor muscles in man. J Physiol. 1984 Oct;355:587–603. doi: 10.1113/jphysiol.1984.sp015440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGO V. G., MARTIN W. R., UNNA K. R. A pharmacological study on the Renshaw cell. J Pharmacol Exp Ther. 1960 May;129:61–68. [PubMed] [Google Scholar]
- Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the spinal cord. UCLA Forum Med Sci. 1969;11:231–265. [PubMed] [Google Scholar]
- Maeda M., Maunz R. A., Wilson V. J. Labyrinthine influence on cat forelimb motoneurons. Exp Brain Res. 1975;22(1):69–86. doi: 10.1007/BF00235412. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Kim J. H. Dynamic characteristics of response in a vestibulomotor reflex. J Neurophysiol. 1969 Jul;32(4):485–495. doi: 10.1152/jn.1969.32.4.485. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Katz R., Bussel B. Influence of voluntary movement and posture on recurrent inhibition in human subjects. Brain Res. 1977 Apr 1;124(3):427–436. doi: 10.1016/0006-8993(77)90944-1. [DOI] [PubMed] [Google Scholar]
- Pompeiano O., Wand P., Srivastava U. C. Responses of Renshaw cells coupled with hindlimb extensor motoneurons to sinusoidal stimulation of labyrinth receptors in the decerebrate cat. Pflugers Arch. 1985 Mar;403(3):245–257. doi: 10.1007/BF00583595. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950 Nov;13(6):395–407. doi: 10.1152/jn.1950.13.6.395. [DOI] [PubMed] [Google Scholar]
- Suzuki J. I., Goto K., Tokumasu K., Cohen B. Implantation of electrodes near individual vestibular nerve branches in mammals. Ann Otol Rhinol Laryngol. 1969 Aug;78(4):815–826. doi: 10.1177/000348946907800414. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Recurrent interactions between motoneurons of known location in the cervical cord of the cat. J Neurophysiol. 1967 Jul;30(4):661–674. doi: 10.1152/jn.1967.30.4.661. [DOI] [PubMed] [Google Scholar]
- Van Keulen L. Autogenetic recurrent inhibition of individual spinal motoneurones of the cat. Neurosci Lett. 1981 Feb 6;21(3):297–300. doi: 10.1016/0304-3940(81)90220-2. [DOI] [PubMed] [Google Scholar]


