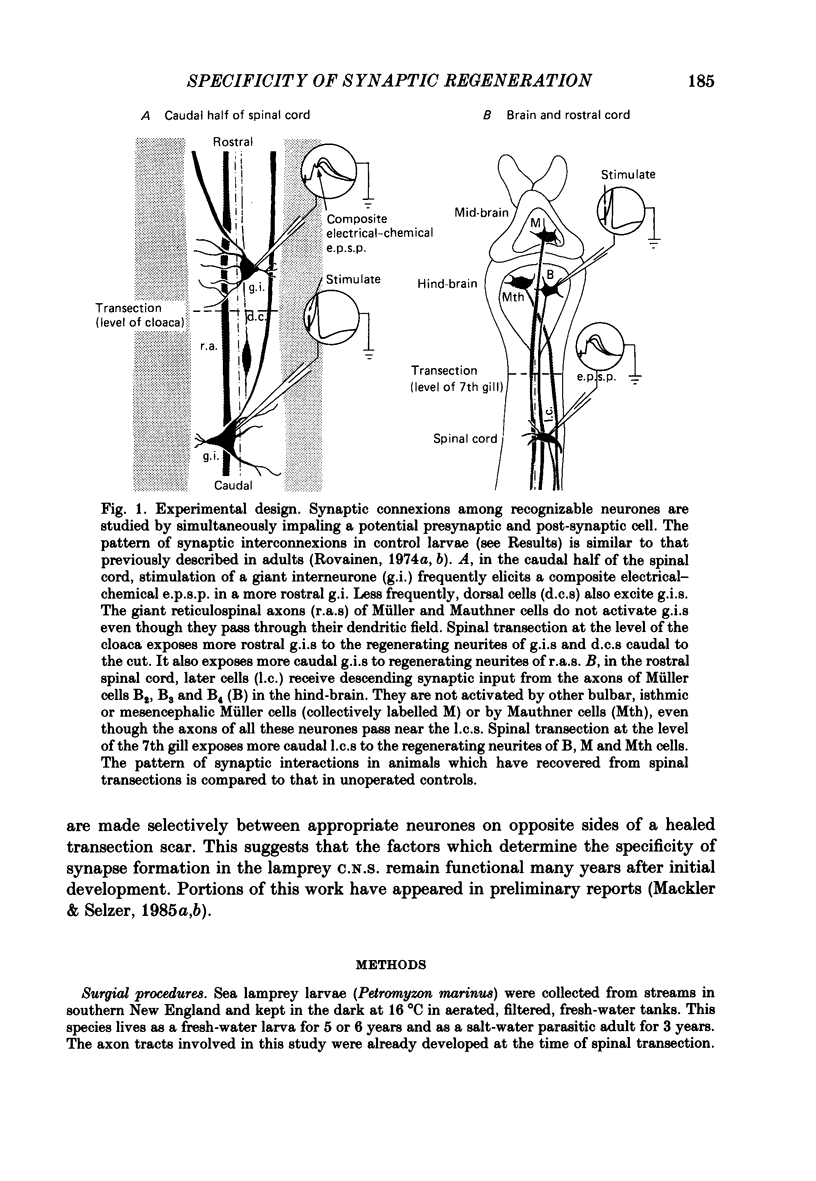
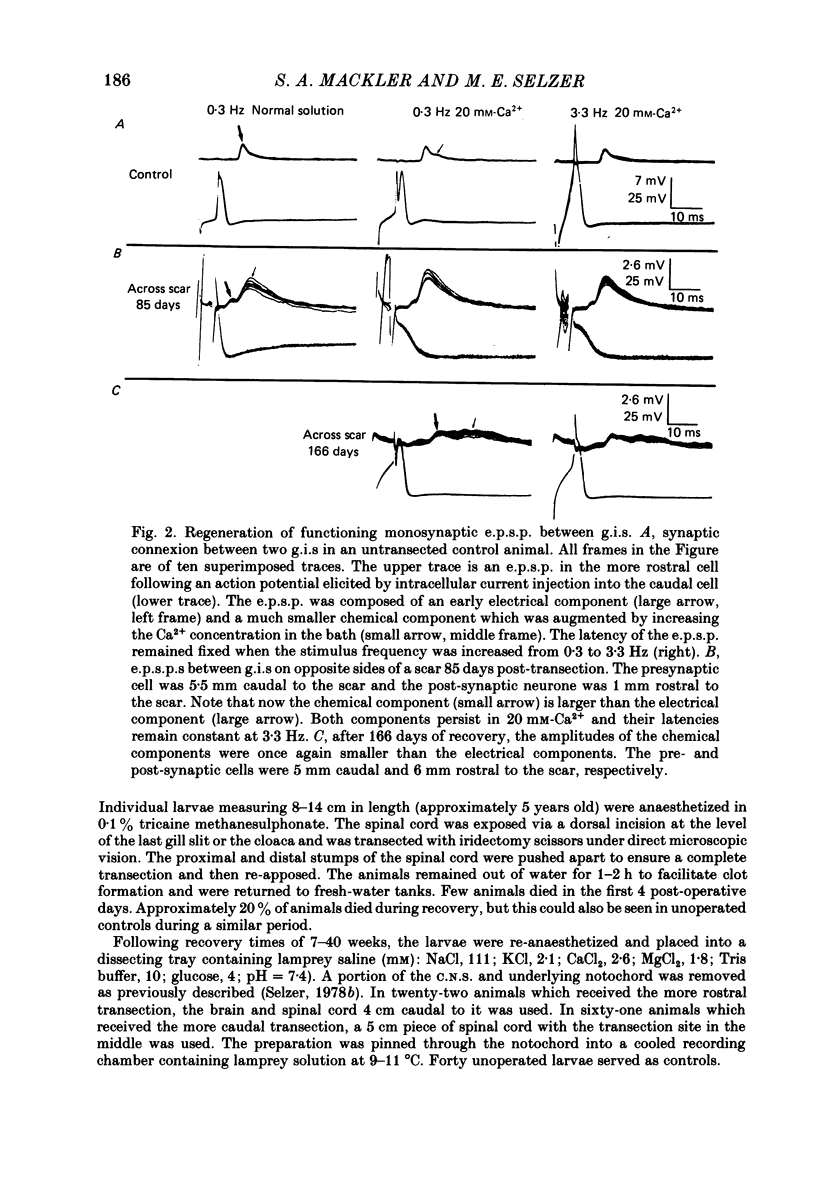
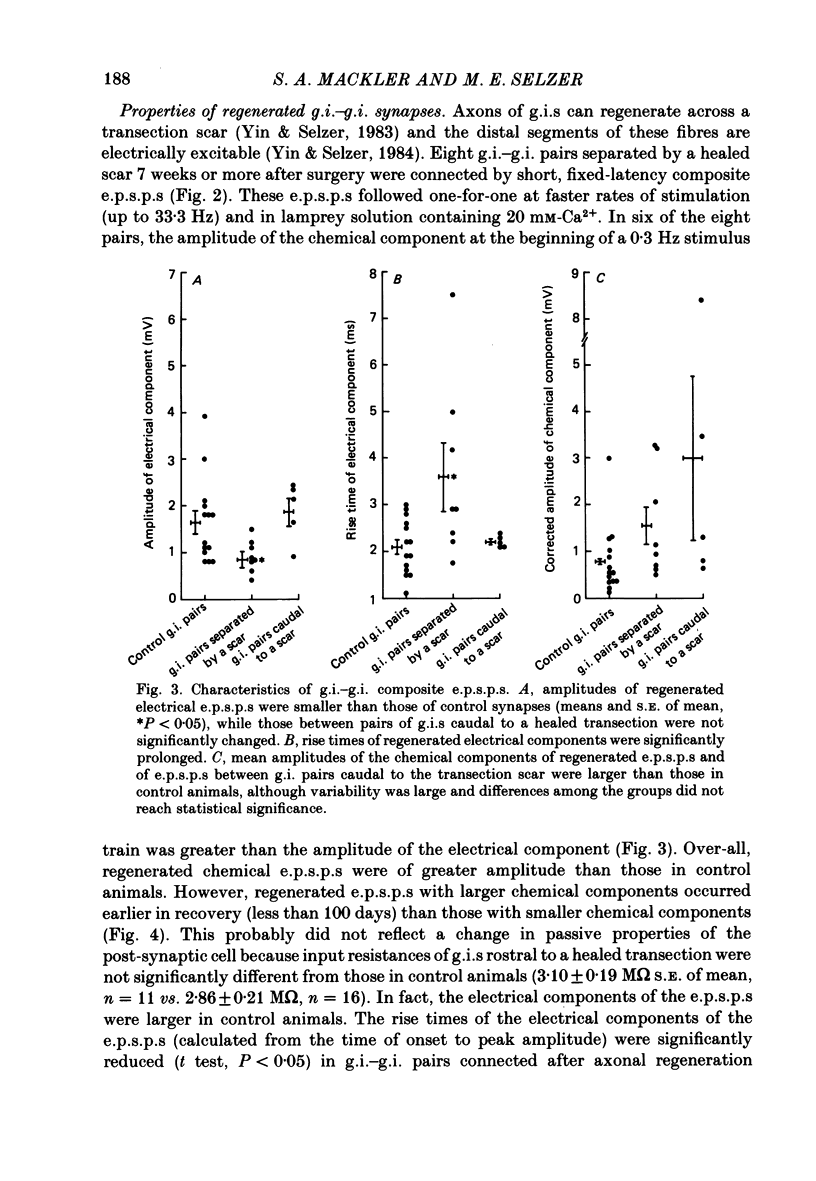
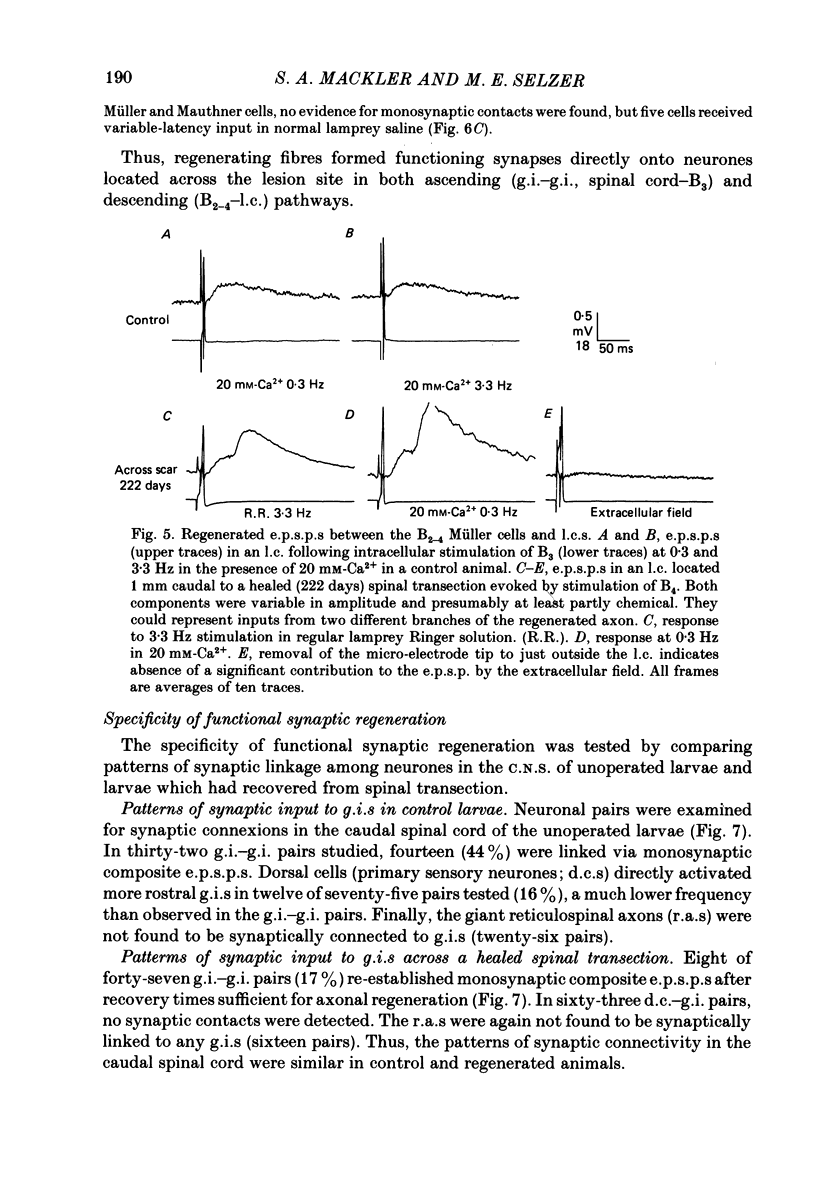
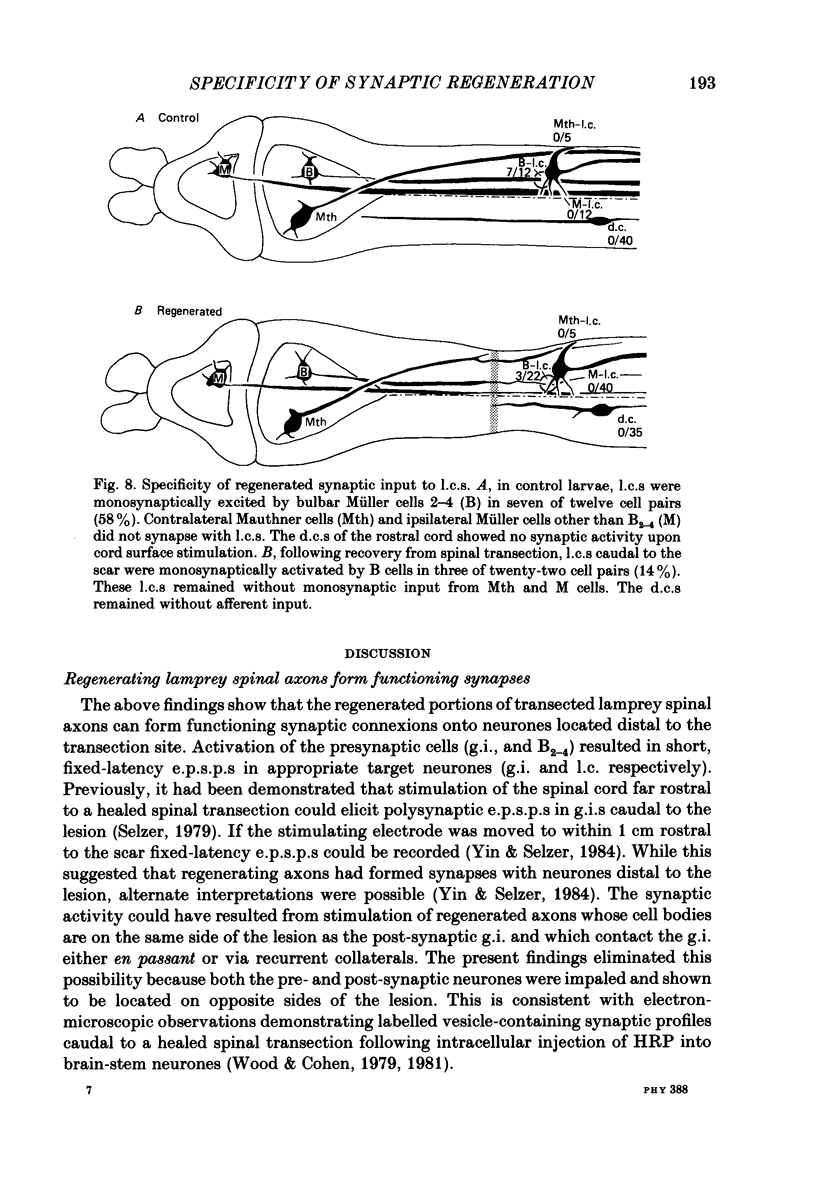
Abstract
1. Pairs of central neurones in large larval sea lampreys were impaled with micro-electrodes and studied for synaptic connexions in both unoperated control animals and animals which had recovered from complete spinal transection. Two identified classes of neurones served as post-synaptic targets: giant interneurones (g.i.s) and lateral cells (l.c.s). Several identified neurone types were tested as potential sources of presynaptic input. 2. When synaptic potentials had short, fixed latencies they also persisted during activation of the presynaptic cell at 3.3-33.3 Hz and were not eliminated after addition of lamprey saline containing high (20 mM) Ca2+. These presumably represented monosynaptic connexions. Variable-latency responses were eliminated by faster rates of stimulation of the presynaptic cell and were mediated via polysynaptic pathways. 3. In control animals, g.i.s received monosynaptic input from more caudal g.i.s in fourteen of thirty-two tested cell pairs. These excitatory post-synaptic potentials (e.p.s.p.s) were composite electrochemical responses. The amplitudes of the earlier electrical component averaged 1.66 +/- 0.24 mV (mean +/- S.E. of mean) and the amplitude of the later chemical component averaged 0.79 +/- 0.05 mV. 4. In operated larvae, eight of forty-seven g.i.-g.i. pairs separated by the transection scar were connected by monosynaptic composite e.p.s.p.s. In these pairs the electrical component averaged 0.84 +/- 0.17 mV (P less than 0.05 vs. control) and the chemical component averaged 1.56 +/- 0.40 mV. The average conduction velocity between these cells was less than that in control g.i.-g.i. pairs (0.93 +/- 0.11 vs. 1.61 +/- 0.25 m/s; P less than 0.01). 5. The l.c.s showed monosynaptic e.p.s.p.s after activation of a subset of the bulbar Müller neurones (B2-4) in seven of twelve pairs. In behaviourally recovered larvae three of twenty-two similar pairs separated by the transection scar were also connected via monosynaptic e.p.s.p.s. The average conduction velocity between these experimental neurones was also less than that in control bulbar-l.c. pairs (1.03 +/- 0.03 vs. 1.58 +/- 0.09 m/s; P less than 0.001). 6. Several types of neurones were either infrequently linked or never connected to g.i.s or to l.c.s in control larvae. In animals which had recovered from a spinal transection, no synaptic connexions were found from such neurones on to g.i.s or l.c.s respectively, in 124 tested cell pairs. In addition, dorsal cells (intraspinal primary sensory neurones) received no synaptic input upon stimulation of the spinal cord before or after transection.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI D. G., SPERRY R. W. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963 Jan;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- Bernstein J. J., Gelderd J. B. Synaptic reorganization following regeneration of goldfish spinal cord. Exp Neurol. 1973 Nov;41(2):402–410. doi: 10.1016/0014-4886(73)90282-3. [DOI] [PubMed] [Google Scholar]
- CLEMENTE C. D. REGENERATION IN THE VERTEBRATE CENTRAL NERVOUS SYSTEM. Int Rev Neurobiol. 1964;6:257–301. doi: 10.1016/s0074-7742(08)60771-0. [DOI] [PubMed] [Google Scholar]
- Christensen B. N. Morphological correlates of synaptic transmission in lamprey spinal cord. J Neurophysiol. 1976 Mar;39(2):197–212. doi: 10.1152/jn.1976.39.2.197. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Teubl W. P. Localization of synaptic input on dendrites of a lamprey spinal cord neurone from physiological measurements of membrane properties. J Physiol. 1979 Dec;297(0):319–333. doi: 10.1113/jphysiol.1979.sp013042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. H., Mackler S. A., Selzer M. E. Functional regeneration following spinal transection demonstrated in the isolated spinal cord of the larval sea lamprey. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2763–2766. doi: 10.1073/pnas.83.8.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Yip J. W. Formation and elimination of foreign synapses on adult salamander muscle. J Physiol. 1978 Jan;274:299–310. doi: 10.1113/jphysiol.1978.sp012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Nicholls J. G., Ready D. F. Membrane properties and selective connexions of identified leech neurones in culture. J Physiol. 1981 Jul;316:203–223. doi: 10.1113/jphysiol.1981.sp013783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H. Retinotopic analysis of fiber pathways in the regenerating retinotectal system of the adult newt cynops Pyrrhogaster. Brain Res. 1981 Feb 9;206(1):27–37. doi: 10.1016/0006-8993(81)90098-6. [DOI] [PubMed] [Google Scholar]
- Homma S., Rovainen C. M. Conductance increases produced by glycine and gamma-aminobutyric acid in lamprey interneurones. J Physiol. 1978 Jun;279:231–252. doi: 10.1113/jphysiol.1978.sp012342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Regeneration and changes in synaptic connections between individual nerve cells in the central nervous system of the leech. Proc Natl Acad Sci U S A. 1972 Mar;69(3):636–639. doi: 10.1073/pnas.69.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. T. Regeneration and functional reconnection of an identified vertebrate central neuron. J Neurosci. 1982 Dec;2(12):1793–1811. doi: 10.1523/JNEUROSCI.02-12-01793.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Mackler S. A., Selzer M. E. Regeneration of functional synapses between individual recognizable neurons in the lamprey spinal cord. Science. 1985 Aug 23;229(4715):774–776. doi: 10.1126/science.2992085. [DOI] [PubMed] [Google Scholar]
- Mackler S. A., Yin H. S., Selzer M. E. Determinants of directional specificity in the regeneration of lamprey spinal axons. J Neurosci. 1986 Jun;6(6):1814–1821. doi: 10.1523/JNEUROSCI.06-06-01814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R., Mart P. E. The mechanism of selective reinnervation of fish eye muscles. IV. Identification of repressed synapses. Brain Res. 1972 Nov 13;46:149–157. doi: 10.1016/0006-8993(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Martin A. R., Wickelgren W. O., Ber1anek R. Effects of iontophoretically applied drugs on spinal interneurons of the lamprey. J Physiol. 1970 May;207(3):653–665. doi: 10.1113/jphysiol.1970.sp009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nja A., Purves D. Re-innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Nov;272(3):633–651. doi: 10.1113/jphysiol.1977.sp012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Thompson W., Yip J. W. Re-innervation of ganglia transplanted to the neck from different levels of the guinea-pig sympathetic chain. J Physiol. 1981;313:49–63. doi: 10.1113/jphysiol.1981.sp013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringham G. L. Localization and electrical characteristics of a giant synapse in the spinal cord of the lamprey. J Physiol. 1975 Oct;251(2):395–407. doi: 10.1113/jphysiol.1975.sp011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovainen C. M. Regeneration of Müller and Mauthner axons after spinal transection in larval lampreys. J Comp Neurol. 1976 Aug 15;168(4):545–554. doi: 10.1002/cne.901680407. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Synaptic interactions of identified nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974 Mar 15;154(2):189–206. doi: 10.1002/cne.901540206. [DOI] [PubMed] [Google Scholar]
- Rovainen C. M. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J Comp Neurol. 1974 Mar 15;154(2):207–223. doi: 10.1002/cne.901540207. [DOI] [PubMed] [Google Scholar]
- Sah D. W., Frank E. Regeneration of sensory-motor synapses in the spinal cord of the bullfrog. J Neurosci. 1984 Nov;4(11):2784–2791. doi: 10.1523/JNEUROSCI.04-11-02784.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S. S. Reinnervation of the extraocular muscles in goldfish is nonselective. J Neurosci. 1986 Mar;6(3):764–773. doi: 10.1523/JNEUROSCI.06-03-00764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer M. E. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol. 1978 Apr;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer M. E. The action of phenytoin on a composite electrical-chemical synapse in the lamprey spinal cord. Ann Neurol. 1978 Mar;3(3):202–206. doi: 10.1002/ana.410030304. [DOI] [PubMed] [Google Scholar]
- Selzer M. E. Variability in maps of identified neurons in the sea lamprey spinal cord examined by a wholemount technique. Brain Res. 1979 Mar 16;163(2):181–193. doi: 10.1016/0006-8993(79)90348-2. [DOI] [PubMed] [Google Scholar]
- Singer M., Nordlander R. H., Egar M. Axonal guidance during embryogenesis and regeneration in the spinal cord of the newt: the blueprint hypothesis of neuronal pathway patterning. J Comp Neurol. 1979 May 1;185(1):1–21. doi: 10.1002/cne.901850102. [DOI] [PubMed] [Google Scholar]
- Sperry R. W., Arora H. L. Selectivity in regeneration of the oculomotor nerve in the cichlid fish, Astronotus ocellatus. J Embryol Exp Morphol. 1965 Dec;14(3):307–317. [PubMed] [Google Scholar]
- WINDLE W. F. Regeneration of axons in the vertebrate central nervous system. Physiol Rev. 1956 Oct;36(4):427–440. doi: 10.1152/physrev.1956.36.4.427. [DOI] [PubMed] [Google Scholar]
- Wigston D. J., Sanes J. R. Selective reinnervation of intercostal muscles transplanted from different segmental levels to a common site. J Neurosci. 1985 May;5(5):1208–1221. doi: 10.1523/JNEUROSCI.05-05-01208.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M. R., Cohen M. J. Synaptic regeneration and glial reactions in the transected spinal cord of the lamprey. J Neurocytol. 1981 Feb;10(1):57–79. doi: 10.1007/BF01181745. [DOI] [PubMed] [Google Scholar]
- Wood M. R., Cohen M. J. Synaptic regeneration in identified neurons of the lamprey spinal cords. Science. 1979 Oct 19;206(4416):344–347. doi: 10.1126/science.482943. [DOI] [PubMed] [Google Scholar]
- Yin H. S., Mackler S. A., Selzer M. E. Directional specificity in the regeneration of lamprey spinal axons. Science. 1984 May 25;224(4651):894–896. doi: 10.1126/science.6719120. [DOI] [PubMed] [Google Scholar]
- Yin H. S., Selzer M. E. Axonal regeneration in lamprey spinal cord. J Neurosci. 1983 Jun;3(6):1135–1144. doi: 10.1523/JNEUROSCI.03-06-01135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. S., Selzer M. E. Electrophysiologic evidence of regeneration of lamprey spinal neurons. Exp Neurol. 1984 Mar;83(3):618–628. doi: 10.1016/0014-4886(84)90128-6. [DOI] [PubMed] [Google Scholar]