Abstract
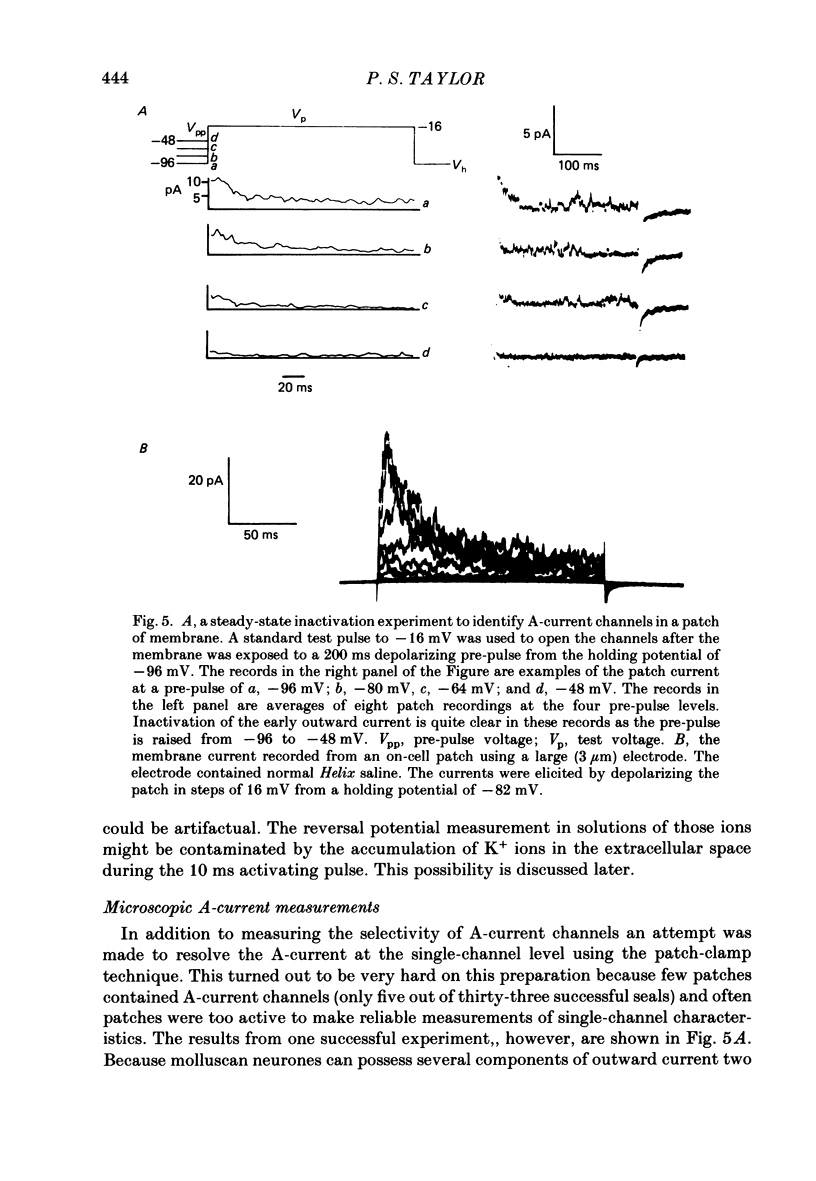
1. The ionic selectivity of A-current K+ channels has been measured in single Helix aspersa neurones by recording the reversal potential shift in test solutions containing various monovalent cations. 2. The A-current channel is permeable to Tl+, K+, Rb+, NH4+ and Cs+. The channels may also be sparingly permeable to Na+ and Li+. Organic cations have an apparent small permeability as judged from their reversal potentials, but this may be an artifact of K+ accumulation. 3. A large patch electrode (3 microns tip) isolated a region that appeared to contain only A-current channels. This may indicate that A-current channels are found in the membrane as rafts of at least 3 microns in diameter. 4. The single-channel conductance calculated from single-channel current steps was 14 pS.
Full text
PDF


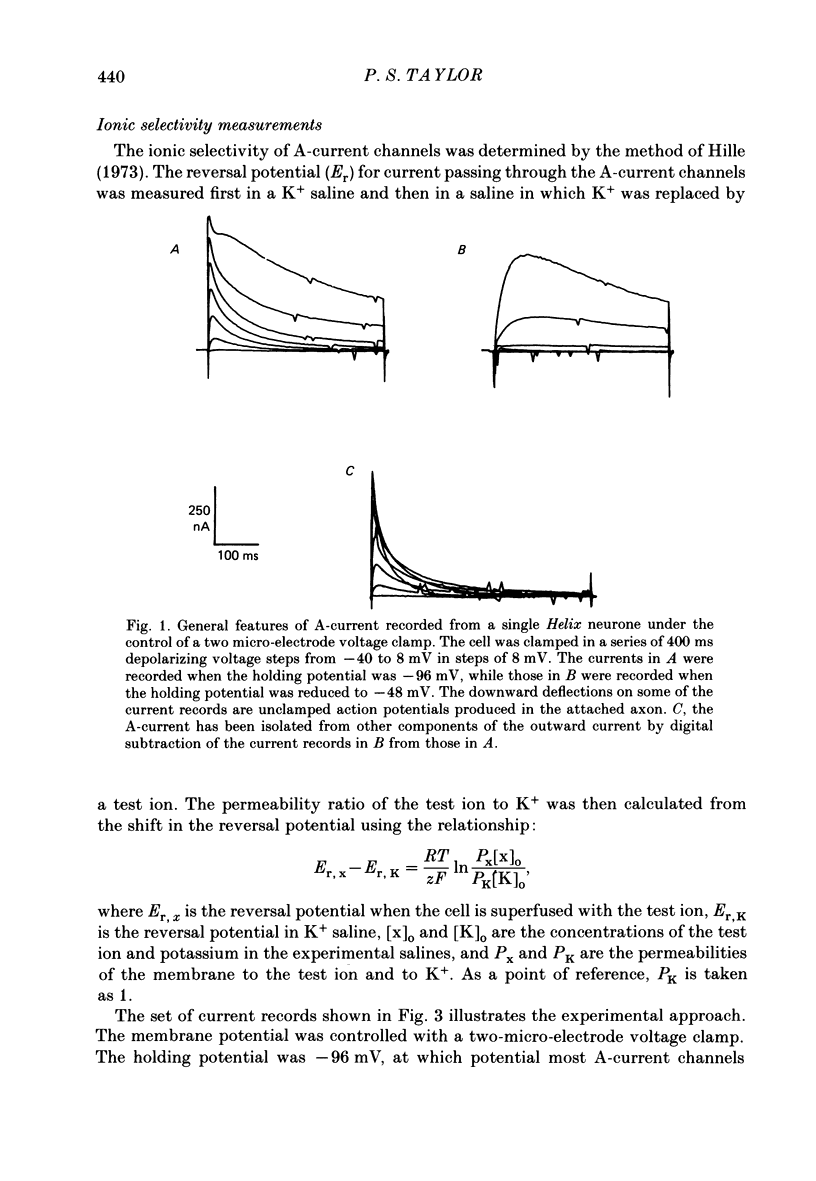
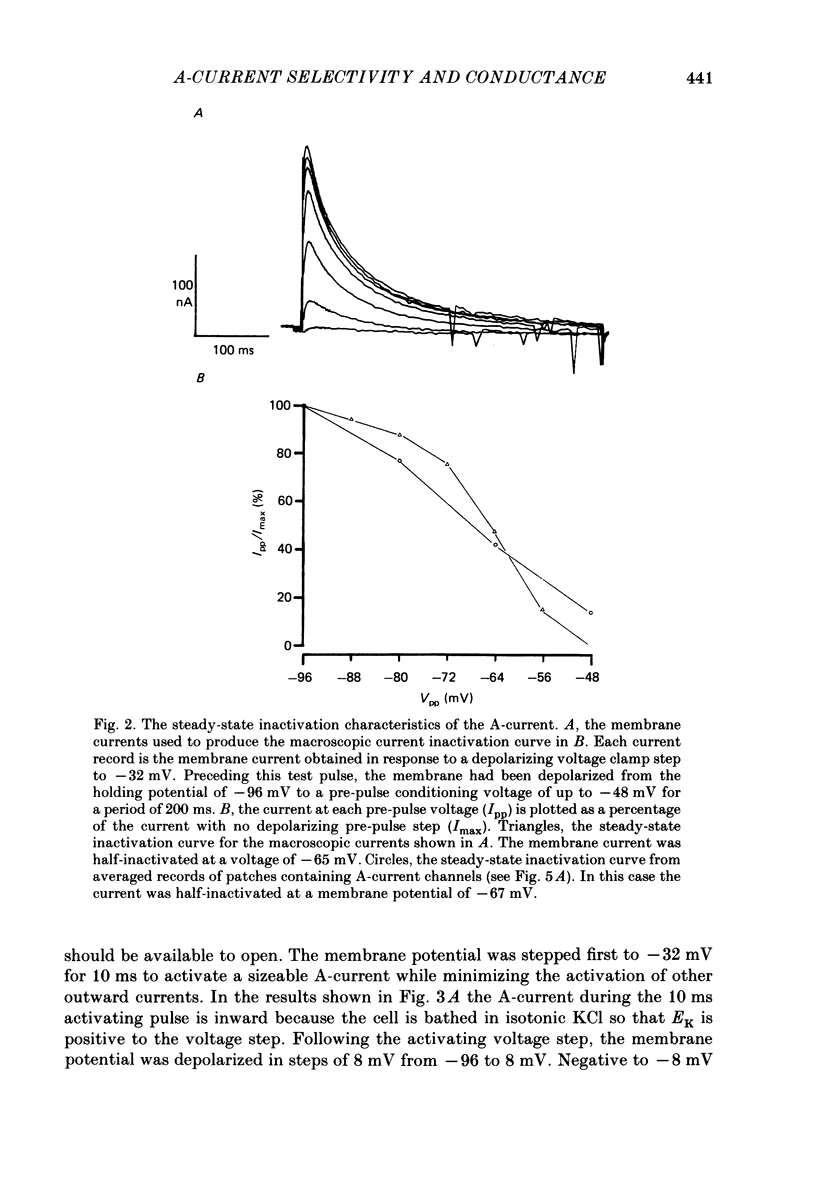
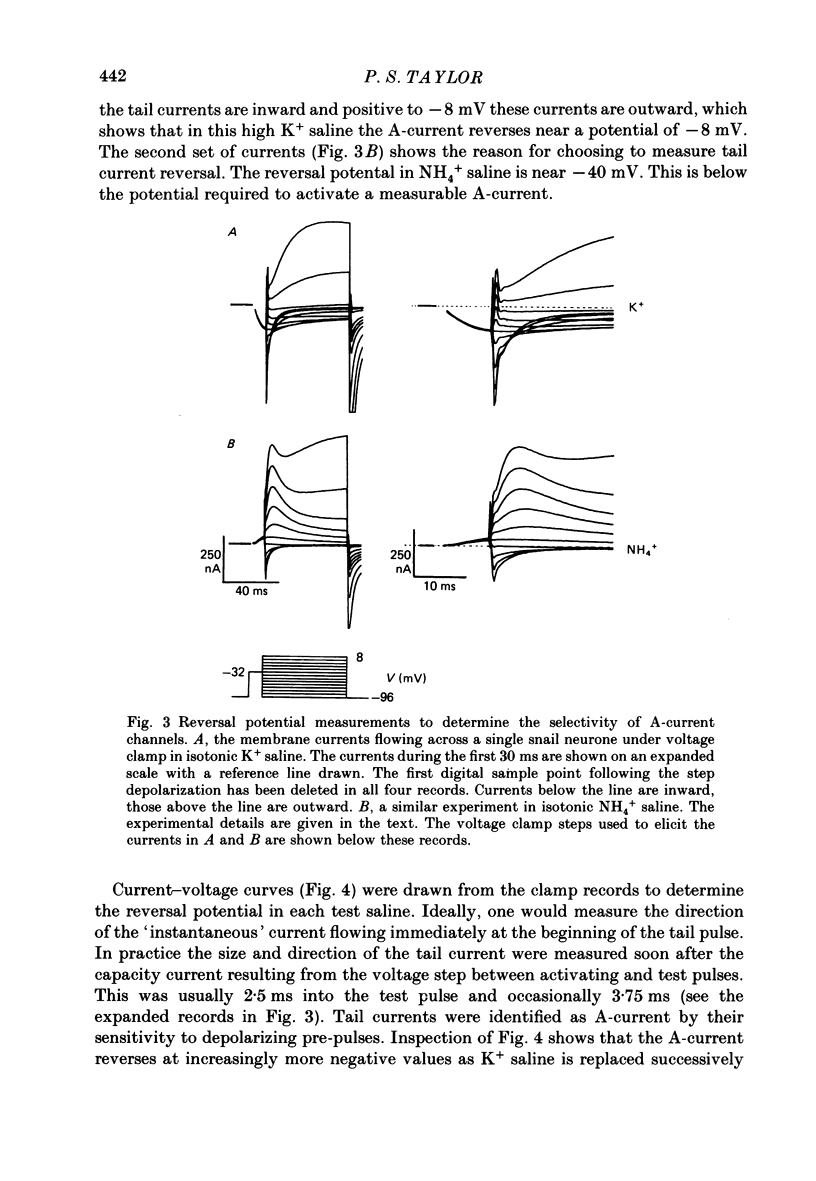
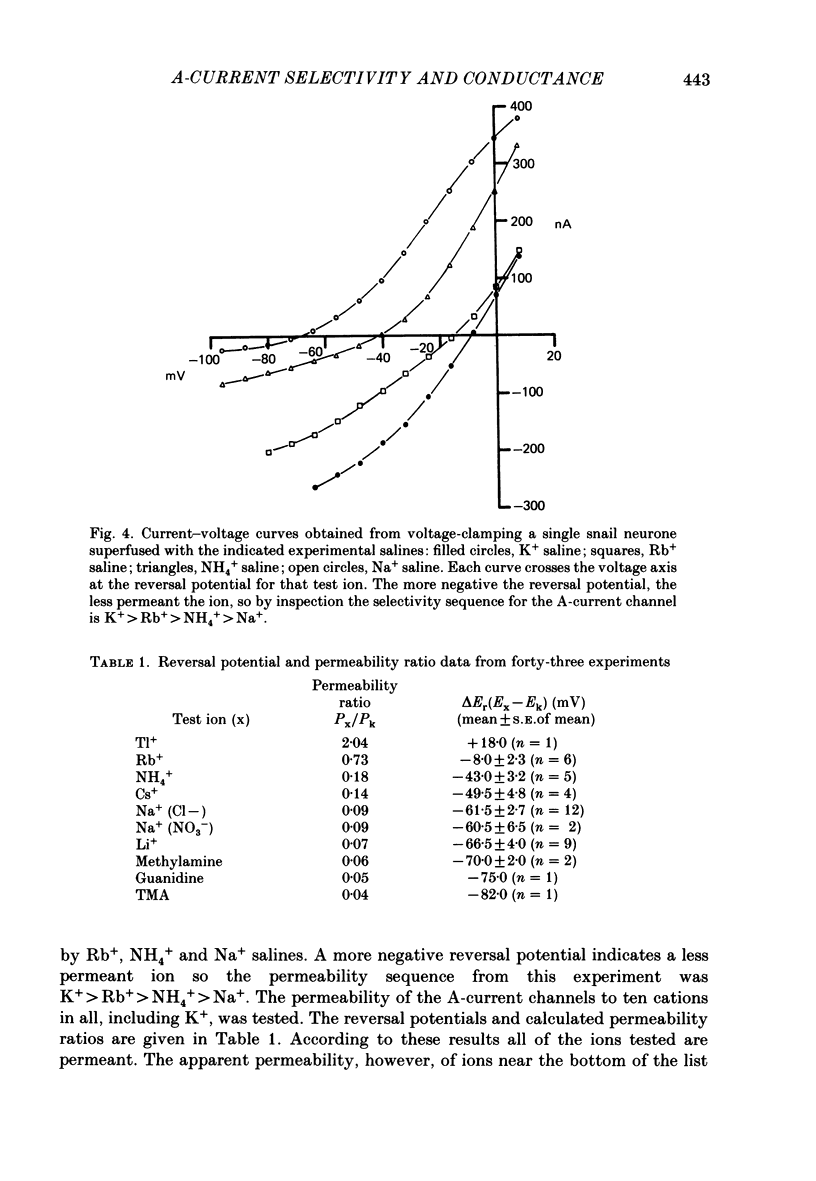



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Almers W., Stirling C. Distribution of transport proteins over animal cell membranes. J Membr Biol. 1984;77(3):169–186. doi: 10.1007/BF01870567. [DOI] [PubMed] [Google Scholar]
- Byrne J. H. Analysis of ionic conductance mechanisms in motor cells mediating inking behavior in Aplysia californica. J Neurophysiol. 1980 Mar;43(3):630–650. doi: 10.1152/jn.1980.43.3.630. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Neural repetitive firing: a comparative study of membrane properties of crustacean walking leg axons. J Neurophysiol. 1975 Jul;38(4):922–932. doi: 10.1152/jn.1975.38.4.922. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Slow repetitive activity from fast conductance changes in neurons. Fed Proc. 1978 Jun;37(8):2139–2145. [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Shrier A. Single-channel analysis of fast transient potassium currents from rat nodose neurones. J Physiol. 1985 Dec;369:199–208. doi: 10.1113/jphysiol.1985.sp015896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Modulation of the excitatory synaptic response by fast transient K+ current in snail neurones. Nat New Biol. 1973 Dec 19;246(155):193–196. doi: 10.1038/newbio246193a0. [DOI] [PubMed] [Google Scholar]
- Dekin M. S., Getting P. A. Firing pattern of neurons in the nucleus tractus solitarius: modulation by membrane hyperpolarization. Brain Res. 1984 Dec 17;324(1):180–184. doi: 10.1016/0006-8993(84)90640-1. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Poo M. Development, maintenance, and modulation of patterned membrane topography: models based on the acetylcholine receptor. Curr Top Dev Biol. 1982;17(Pt 3):77–100. doi: 10.1016/s0070-2153(08)60519-0. [DOI] [PubMed] [Google Scholar]
- Getting P. A. Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol. 1983 Apr;49(4):1036–1050. doi: 10.1152/jn.1983.49.4.1036. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kazachenko V. N., Geletyuk V. I. The potential-dependent K+ channel in molluscan neurones is organized in a cluster of elementary channels. Biochim Biophys Acta. 1984 Jun 13;773(1):132–142. doi: 10.1016/0005-2736(84)90558-3. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Nakajima S. Analysis of K inactivation and TEA action in the supramedullary cells of puffer. J Gen Physiol. 1966 Mar;49(4):629–640. doi: 10.1085/jgp.49.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Differential action of TEA + on two K + -current componentss of a molluscan neurone. Pflugers Arch. 1972;336(2):87–100. doi: 10.1007/BF00592924. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F. Ion conductance and ion selectivity of potassium channels in snail neurones. J Membr Biol. 1980 Dec 15;57(2):103–118. doi: 10.1007/BF01868997. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981 Sep 17;293(5829):228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]


