Abstract
1. Single afferent fibres in the internal branch of the superior laryngeal nerve which responded to light touch or gentle probing of discrete areas of the exposed epithelium of the opened larynx were identified in anaesthetized, paralysed cats (148 fibres) and rabbits (58 fibres). 2. A quantitative examination of the sensitivity of these laryngeal mechanoreceptors to both static (step indentations) and dynamic (vibratory) forms of mechanical stimulation was undertaken using a servo-controlled mechanical stimulator. 3. In both species two predominant classes of mechanoreceptors were observed (Boushey, Richardson, Widdicombe & Wise, 1974). One class was distinguished by a regular and continuous pattern of activity at a frequency of 10-70 Hz (tonic fibres, sixty-six in cat, thirty-five in rabbit). The other class was silent or (more rarely) irregularly active at a very low frequency (silent fibres, eighty-two in cat, twenty-three in rabbit). 4. The location of the receptive fields was determined by manual probing. Inter-species and regional variations in receptive field location were observed for the two fibre groups. 5. Conduction velocity was measured for twenty-one tonic and seven silent fibres in the rabbit by a pre-triggered averaging technique. The results obtained (tonic: range 10.8-30.0, mean +/- S.E. of mean 21.4 +/- 1.2 m/s; silent: 14.8-28.6, 20.4 +/- 1.8 m/s) were characteristic of group III afferent fibres but were not significantly different for the two classes. 6. Both classes of receptor showed a response at the onset of a step indentation of the region of the mucosa that corresponded to their receptive field. Subsequent to this brief initial response the behaviour of the two classes diverged markedly. Tonic fibres were invariably slowly adapting whereas most (forty-four out of fifty-five in cat; twenty-two out of twenty-three in rabbit) silent fibres were rapidly adapting, at least for smaller indentation amplitudes. 7. Receptors of both classes were readily entrained to discharge at the same frequency as the probe stimulator (1:1 entrainment) when this was made to vibrate upon the receptive area for test periods of 0.5 or 1.0 s. Tuning curves were constructed of the minimum amplitudes required to elicit 1:1 entrainment throughout an entire test period at various frequencies. 8. Individual fibres in the two classes could be entrained at frequencies up to 400 Hz or more at sensitive (e.g. less than 100 microns) vibratory amplitudes. However, all fibres were less sensitive at these higher frequencies than at some lower point on the frequency scale.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




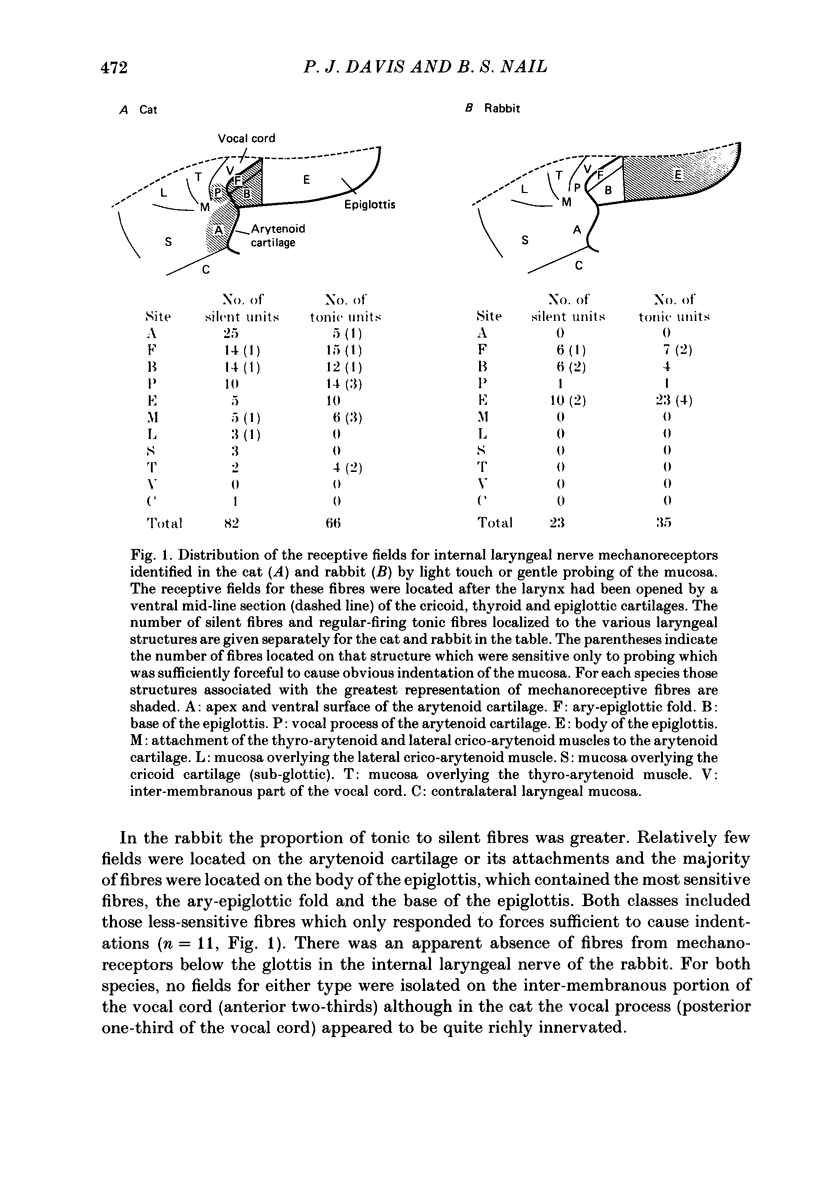

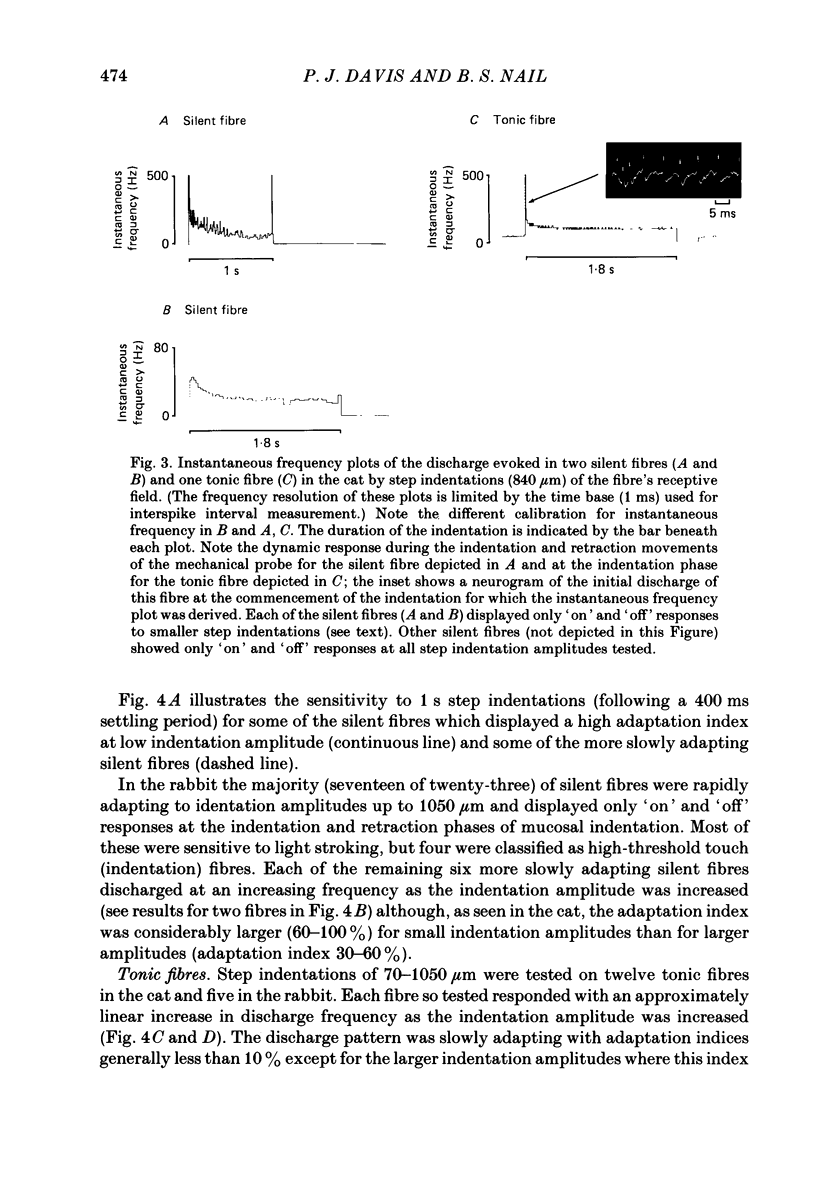
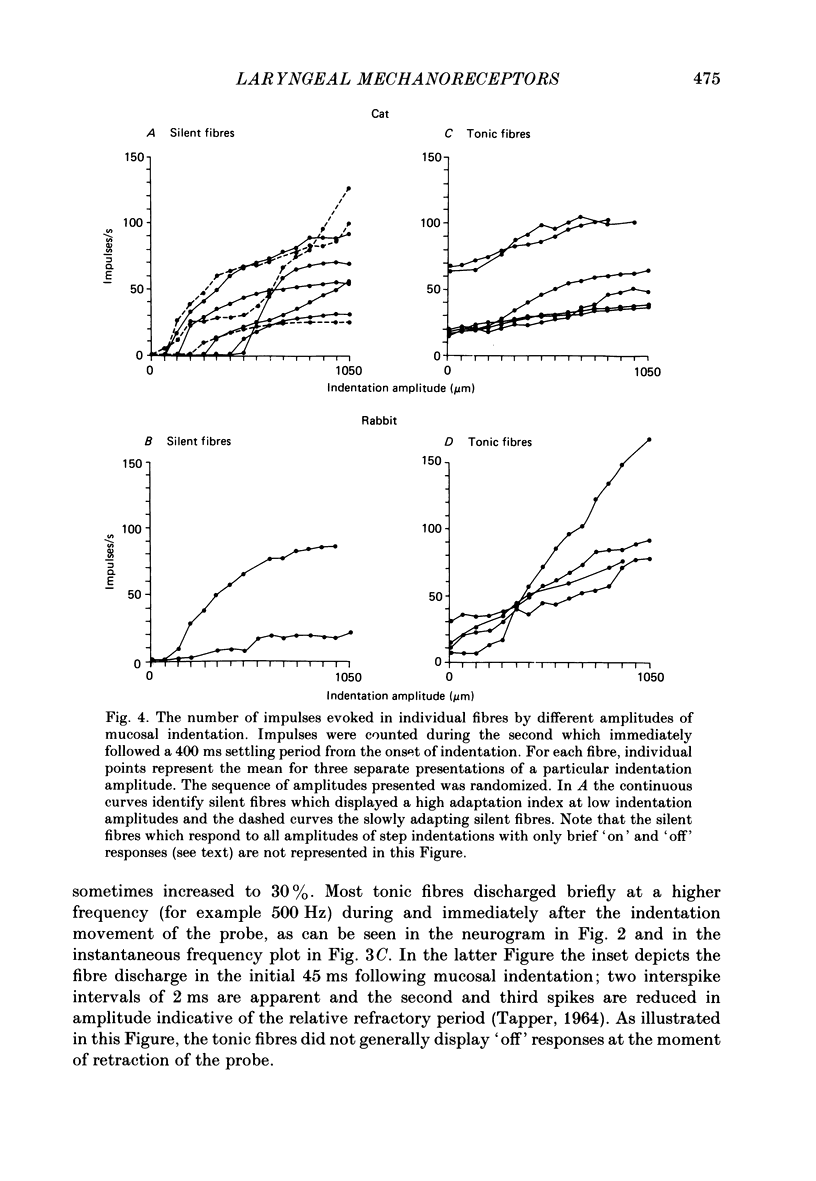

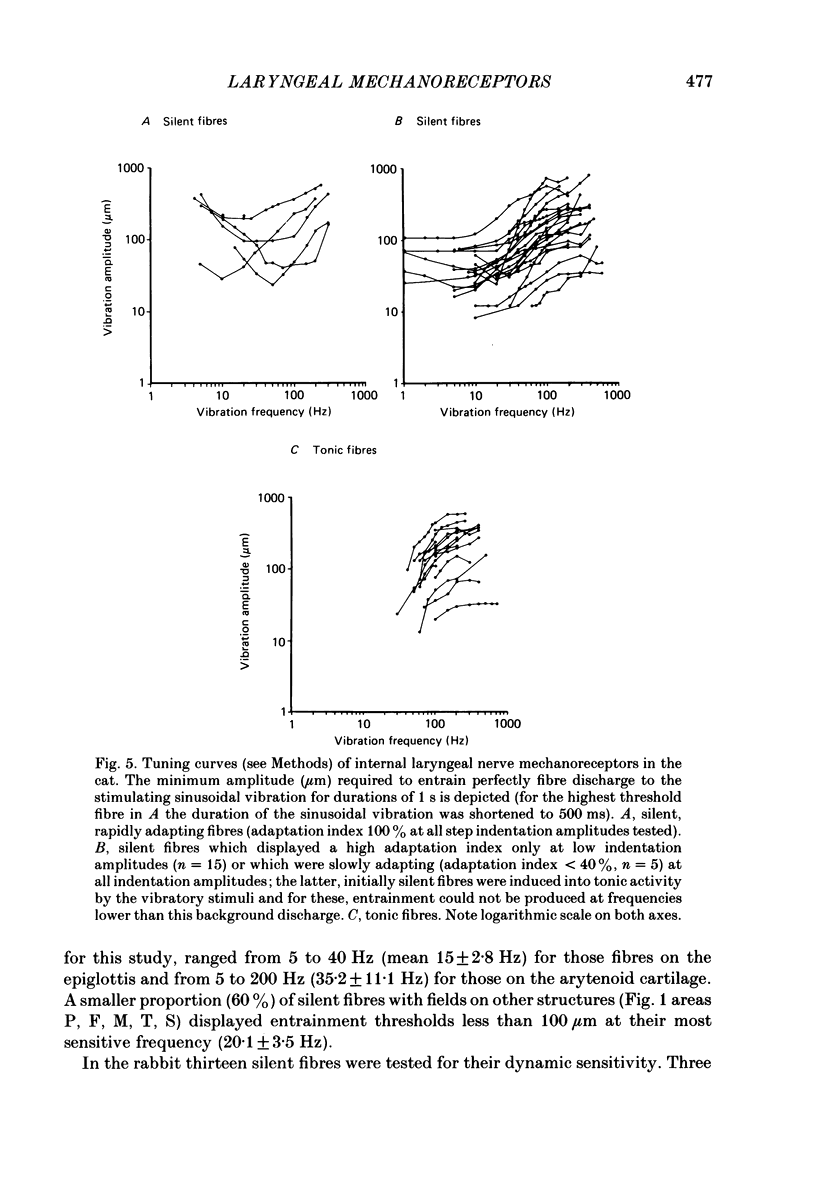







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREW B. L. Proprioception at the joint of the epiglottis of the rat. J Physiol. 1954 Dec 10;126(3):507–523. doi: 10.1113/jphysiol.1954.sp005225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D., Jr, St John W. M. Adaptation of pulmonary stretch receptors in different mammalian species. Respir Physiol. 1979 Aug;37(3):303–312. doi: 10.1016/0034-5687(79)90077-x. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Richardson P. S., Widdicombe J. G., Wise J. C. The response of laryngeal afferent fibres to mechanical and chemical stimuli. J Physiol. 1974 Jul;240(1):153–175. doi: 10.1113/jphysiol.1974.sp010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrzycka E., NAil B. S., Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive "tactile" neurones. J Physiol. 1977 Jun;268(1):251–270. doi: 10.1113/jphysiol.1977.sp011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I., Rowe M. J., Sessle B. J. "Tactile" stimulus intensity: information transmission by relay neurons in different trigeminal nuclei. Science. 1968 May 17;160(3829):791–794. doi: 10.1126/science.160.3829.791. [DOI] [PubMed] [Google Scholar]
- Davies A., Vizek M. Effect of pulses of pressure applied to the larynx of rabbits on their pattern of breathing. Lung. 1982;160(3):157–164. doi: 10.1007/BF02719287. [DOI] [PubMed] [Google Scholar]
- EVANS D. H., MURRAY J. G. Histological and functional studies on the fibre composition of the vagus nerve of the rabbit. J Anat. 1954 Jul;88(3):320–337. [PMC free article] [PubMed] [Google Scholar]
- FEINDEL W. The neural pattern of the epiglottis. J Comp Neurol. 1956 Sep;105(2):269–285. doi: 10.1002/cne.901050206. [DOI] [PubMed] [Google Scholar]
- Harding R., Johnson P., McClelland M. E. Liquid-sensitive laryngeal receptors in the developing sheep, cat and monkey. J Physiol. 1978 Apr;277:409–422. doi: 10.1113/jphysiol.1978.sp012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G., Gauthier P., Monteau R. Central respiratory drive and recruitment order of phrenic and inspiratory laryngeal motoneurones. Respir Physiol. 1983 Mar;51(3):341–359. doi: 10.1016/0034-5687(83)90028-2. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., St John W. M., Bartlett D., Jr Receptors responding to changes in upper airway pressure. Respir Physiol. 1984 Mar;55(3):355–366. doi: 10.1016/0034-5687(84)90057-4. [DOI] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K., Korpas J., Widdicombe J. G. Intraepithelial nerve fibres of the cat larynx and the expiration reflex [proceedings]. J Physiol. 1978 Feb;275:35P–36P. [PubMed] [Google Scholar]
- Jänig W. Morphology of rapidly and slowly adapting mechanoreceptors in the hairless skin of the cat's hind foot. Brain Res. 1971 May 7;28(2):217–231. doi: 10.1016/0006-8993(71)90656-1. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- MURRAY J. G. Innervation of the intrinsic muscles of the cat's larynx by the recurrent laryngeal nerve: a unimodal nerve. J Physiol. 1957 Jan 23;135(1):206–212. doi: 10.1113/jphysiol.1957.sp005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew O. P., Sant'Ambrogio G., Fisher J. T., Sant'Ambrogio F. B. Laryngeal pressure receptors. Respir Physiol. 1984 Jul;57(1):113–122. doi: 10.1016/0034-5687(84)90037-9. [DOI] [PubMed] [Google Scholar]
- Mei N., Nourigat B. Etude électrophysiologique des neurones sensitifs du nerf laryngé supérieur. C R Seances Soc Biol Fil. 1968 Jul;162(1):149–153. [PubMed] [Google Scholar]
- Miller A. J., Loizzi R. F. Anatomical and functional differentiation of superior laryngeal nerve fibers affecting swallowing and respiration. Exp Neurol. 1974 Feb;42(2):369–387. doi: 10.1016/0014-4886(74)90033-8. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Onodera K. Adaptation of the generator potential in the crayfish stretch receptors under constant length and constant tension. J Physiol. 1969 Jan;200(1):187–204. doi: 10.1113/jphysiol.1969.sp008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESSMAN J. J., KELEMEN G. Physiology of the larynx. Physiol Rev. 1955 Jul;35(3):506–554. doi: 10.1152/physrev.1955.35.3.506. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Some aspects of the control of cricothyroid muscle activity. Arch Int Physiol Biochim. 1966 Feb;74(1):154–168. doi: 10.3109/13813456609059898. [DOI] [PubMed] [Google Scholar]
- SAMPSON S., EYZAGUIRRE C. SOME FUNCTIONAL CHARACTERISTICS OF MECHANORECEPTORS IN THE LARYNX OF THE CAT. J Neurophysiol. 1964 May;27:464–480. doi: 10.1152/jn.1964.27.3.464. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio F. B., Fisher J. T., Sant'Ambrogio G. Adaptation of airway stretch receptors in newborn and adult dogs. Respir Physiol. 1983 Jun;52(3):361–369. doi: 10.1016/0034-5687(83)90091-9. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G., Mathew O. P., Fisher J. T., Sant'Ambrogio F. B. Laryngeal receptors responding to transmural pressure, airflow and local muscle activity. Respir Physiol. 1983 Dec;54(3):317–330. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Sessle B. J. Presynaptic excitability changes induced in single laryngeal primary afferent fibres. Brain Res. 1973 Apr 27;53(2):333–342. doi: 10.1016/0006-8993(73)90218-7. [DOI] [PubMed] [Google Scholar]
- Shingai T. Ionic mechanism of water receptors in the laryngeal mucosa of the rabbit. Jpn J Physiol. 1977;27(1):27–42. doi: 10.2170/jjphysiol.27.27. [DOI] [PubMed] [Google Scholar]
- Storey A. T. A functional analysis of sensory units innervating epiglottis and larynx. Exp Neurol. 1968 Mar;20(3):366–383. doi: 10.1016/0014-4886(68)90080-0. [DOI] [PubMed] [Google Scholar]
- TAPPER D. N. CUTANEOUS SLOWLY ADAPTING MECHANORECEPTORS IN THE CAT. Science. 1964 Jan 3;143(3601):53–54. doi: 10.1126/science.143.3601.53. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Receptors in the trachea and bronchi of the cat. J Physiol. 1954 Jan;123(1):71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]


