Abstract
Concentrations of phosphorus metabolites and intracellular pH have been measured in non-pregnant, late-pregnant and post-partum rat uterus using 31P nuclear magnetic resonance (31P n.m.r.). Intact uterine tissue was superfused with oxygenated de-Jalon solution at 4, 20 or 37 degrees C while inside the n.m.r. spectrometer. The phosphocreatine concentration [PCr], was higher and the inorganic phosphate concentration [Pi], lower than values determined by chemical analysis of extracts from both pregnant and non-pregnant rat uterus. [PCr] was 1.4-fold greater in late-pregnant than in non-pregnant rat uterus. Following parturition, large changes were observed in [PCr], [Pi] and in an unidentified metabolite in the phosphomonoester (PME) region of the n.m.r. spectrum. The time course of the recovery of these metabolites to prepregnant values was determined. The [PCr] remained below the non-pregnant value for at least 1 week post-partum and the [Pi] was elevated, compared to the non-pregnant value, during this period. More rapid changes were seen in the [PME], which doubled on day 0 post-partum but almost returned to its non-pregnant value on day 1 post-partum. No significant difference was observed between intracellular pH values in late-pregnant and non-pregnant rat uterus; however, there was a large acid shift following parturition. Intracellular pH depended upon the temperature at which the tissue was maintained. The effect of muscular work during parturition was investigated by comparing Caesarian-sectioned uteri with uteri which had undergone normal parturition. Uteri examined 1 day after Caesarian operation showed no differences in metabolite levels from normal, 1 day post-partum uteri. We conclude that concentrations of phosphorus metabolites depend upon the physiological state of the uterus. We suggest that the changes following parturition are not a consequence of the mechanical work performed by the uterus, but must be caused by some other event associated with parturition such as hormonal changes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Direct measurement of intracellular pH and buffering power in smooth muscle cells of guinea-pig vas deferens. J Physiol. 1984 Apr;349:571–585. doi: 10.1113/jphysiol.1984.sp015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W., Dietz V., Quintern J. Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. J Physiol. 1984 Dec;357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenton D. P., Garrod P. J., Krywawych S., Reynolds E. O., Bachelard H. S., Cox D. W., Morris P. G. Phosphoethanolamine is major constituent of phosphomonester peak detected by 31P NMR in newborn brain. Lancet. 1985 Jan 12;1(8420):115–115. doi: 10.1016/s0140-6736(85)92012-4. [DOI] [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Nuclear magnetic resonance spectra of adenosine di- and triphosphate. II. Effect of complexing with divalent metal ions. J Biol Chem. 1962 Jan;237:176–181. [PubMed] [Google Scholar]
- CRETIUS K. Der Kreatinphosphatgehalt menschlicher Skelett- und Uterusmuskulatur. Z Geburtshilfe Gynakol. 1957;149(2):113–122. [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J. Quantitative analysis of metabolite levels in normal human subjects by 31P topical magnetic resonance. Biosci Rep. 1982 Sep;2(9):727–733. doi: 10.1007/BF01114836. [DOI] [PubMed] [Google Scholar]
- Degani H., Shaer A., Victor T. A., Kaye A. M. Estrogen-induced changes in high-energy phosphate metabolism in rat uterus: 31P NMR studies. Biochemistry. 1984 Jun 5;23(12):2572–2577. doi: 10.1021/bi00307a006. [DOI] [PubMed] [Google Scholar]
- FERRARI V., HARKNESS R. D. Free amino-acids in liver and blood after partial hepatectomy in normal and adrenalectomized rats. J Physiol. 1954 Jun 28;124(3):443–463. doi: 10.1113/jphysiol.1954.sp005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J. R., Cady E., Edwards R. H., McCready V. R., Wilkie D. R., Wiltshaw E. 31P-NMR studies of a human tumour in situ. Lancet. 1983 Jun 25;1(8339):1435–1436. doi: 10.1016/s0140-6736(83)92375-9. [DOI] [PubMed] [Google Scholar]
- HARKNESS M. L., HARKNESS R. D. The collagen content of the reproductive tract of the rat during pregnancy and lactation. J Physiol. 1954 Mar 29;123(3):492–500. doi: 10.1113/jphysiol.1954.sp005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENKES J. H., CSAPO A. Changes in the adenosine triphosphate and creatine phosphate content of the rabbit uterus throughout sexual maturation and after ovulation. Endocrinology. 1952 Jan;50(1):37–50. doi: 10.1210/endo-50-1-37. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Kellie A. E. The effects of oestradiol on the acid-soluble nucleotides of rat uterus. Biochem J. 1970 Sep;119(2):187–191. doi: 10.1042/bj1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L. M., Gupta R. K., Laragh J. H. Intracellular free magnesium in erythrocytes of essential hypertension: relation to blood pressure and serum divalent cations. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6511–6515. doi: 10.1073/pnas.81.20.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts P., Wray S. Changes in brain phosphorus metabolites during the post-natal development of the rat. J Physiol. 1985 Feb;359:417–429. doi: 10.1113/jphysiol.1985.sp015593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLFIN P., CLAUSER H., GAUTHERON D. Influence of oestradiol and progesterone injections on the acid-soluble phosphate fractions of the rat uterus. Biochim Biophys Acta. 1957 Apr;24(1):137–140. doi: 10.1016/0006-3002(57)90156-7. [DOI] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. The content of adenosinetriphosphate and creatine phosphate in uterine muscle of rats and rabbits. Acta Physiol Scand. 1950;21(1):1–17. doi: 10.1111/j.1748-1716.1950.tb00159.x. [DOI] [PubMed] [Google Scholar]
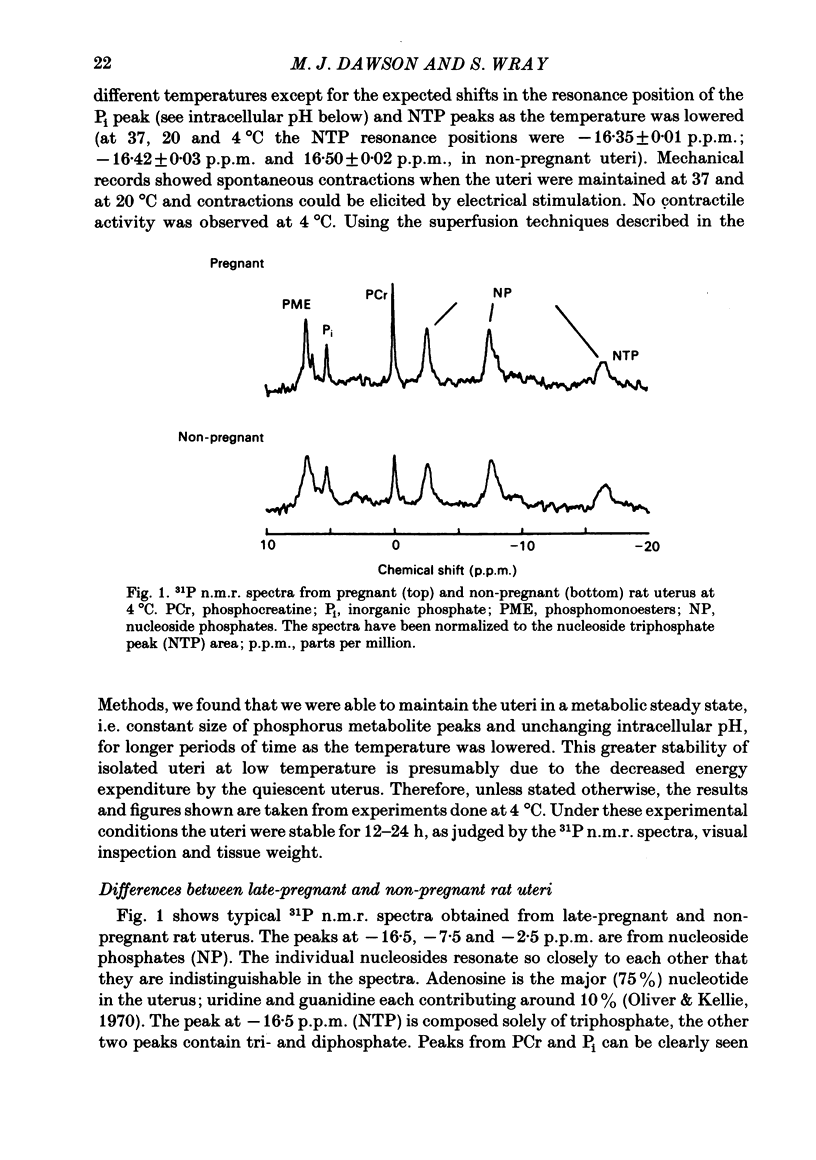
- Wray S. The role of mechanical and hormonal stimuli on uterine involution in the rat. J Physiol. 1982 Jul;328:1–9. doi: 10.1113/jphysiol.1982.sp014249. [DOI] [PMC free article] [PubMed] [Google Scholar]


