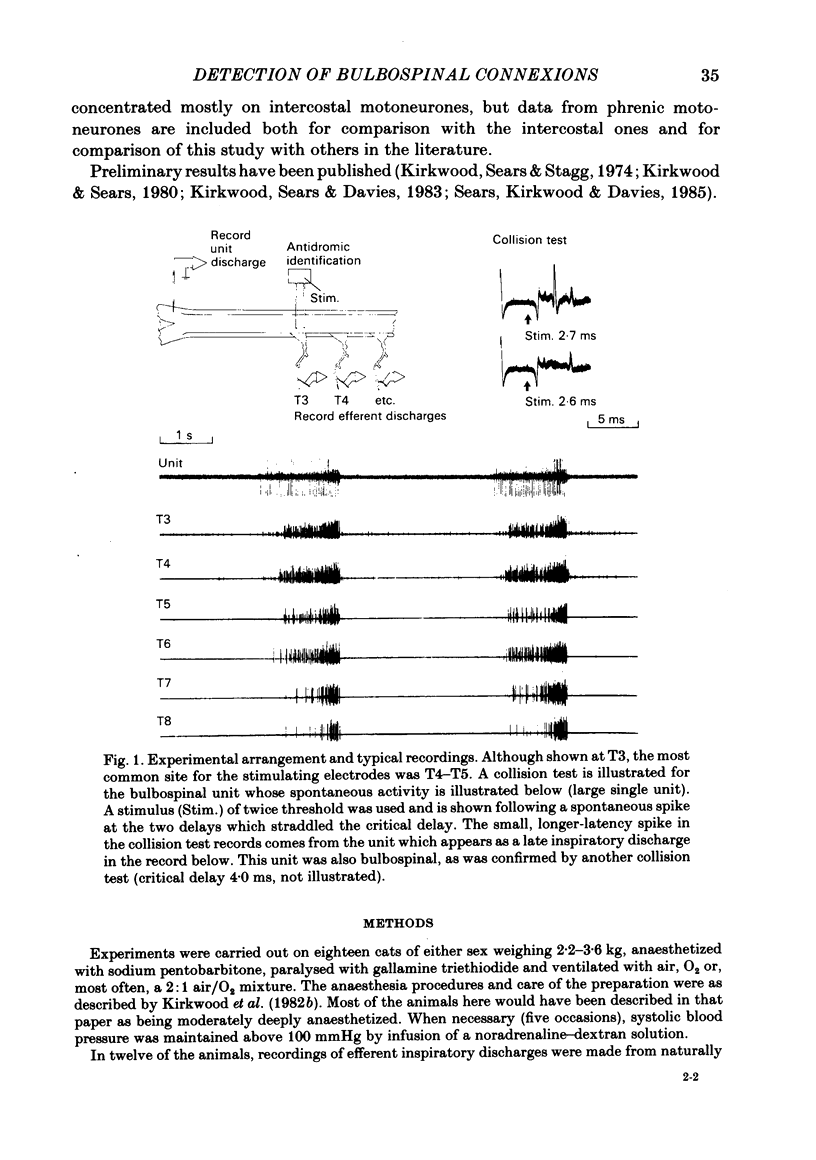
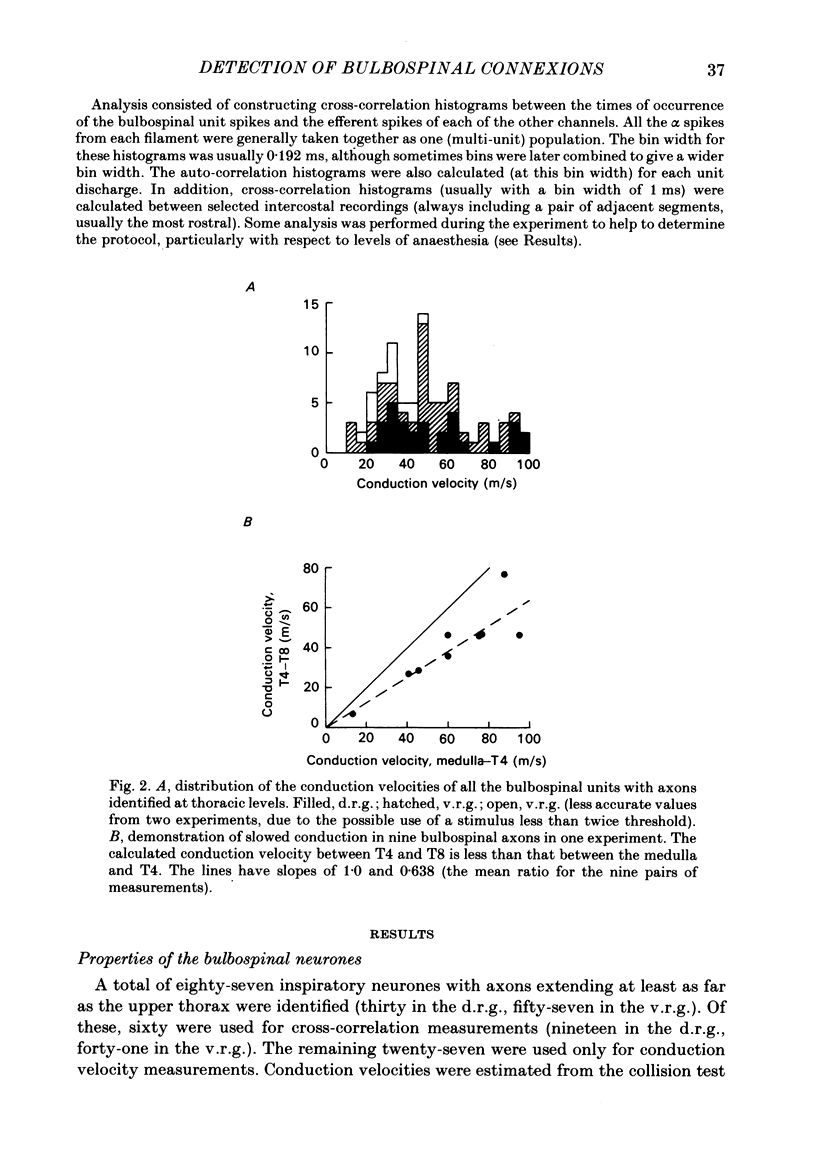
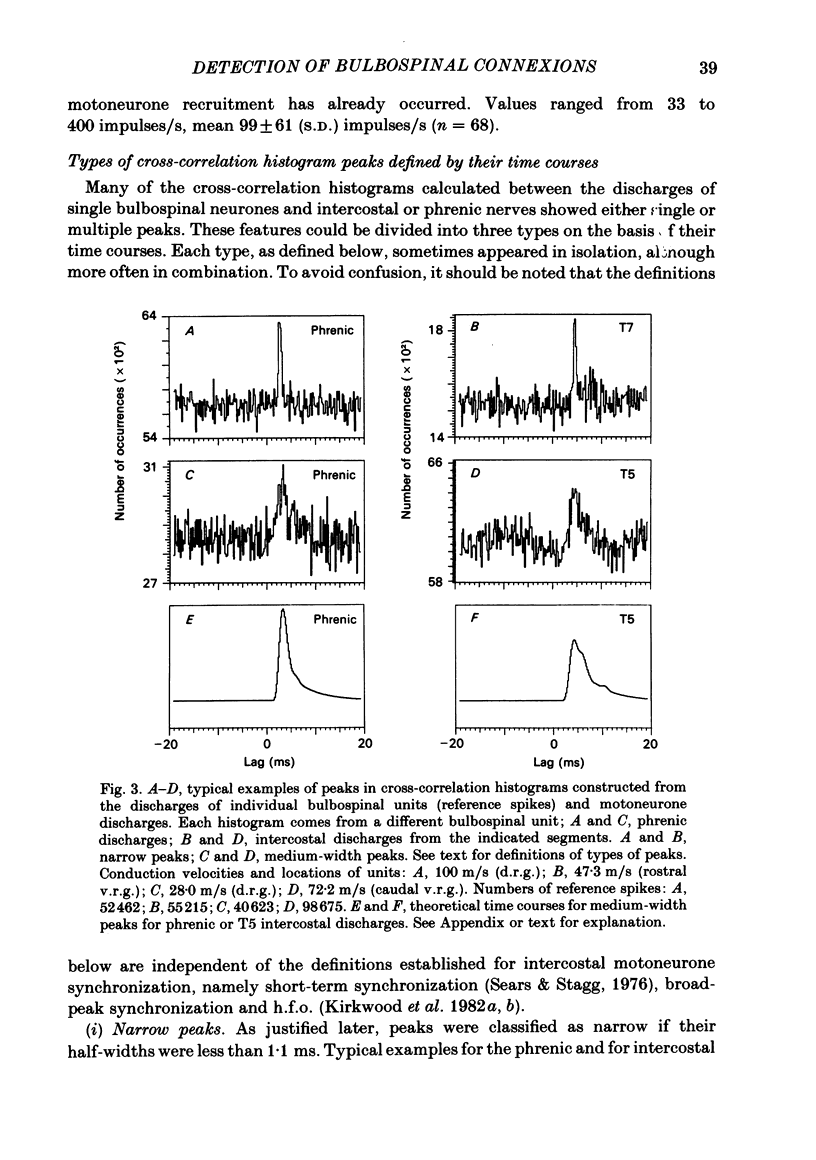
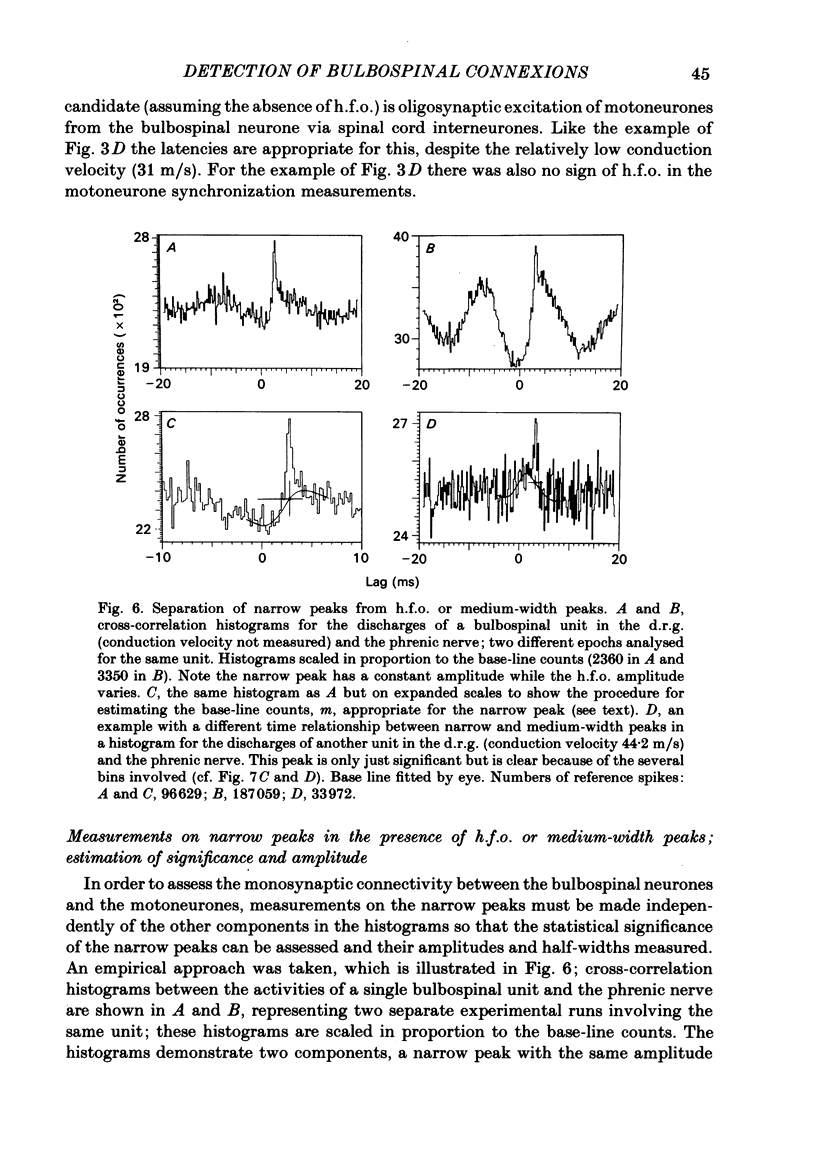
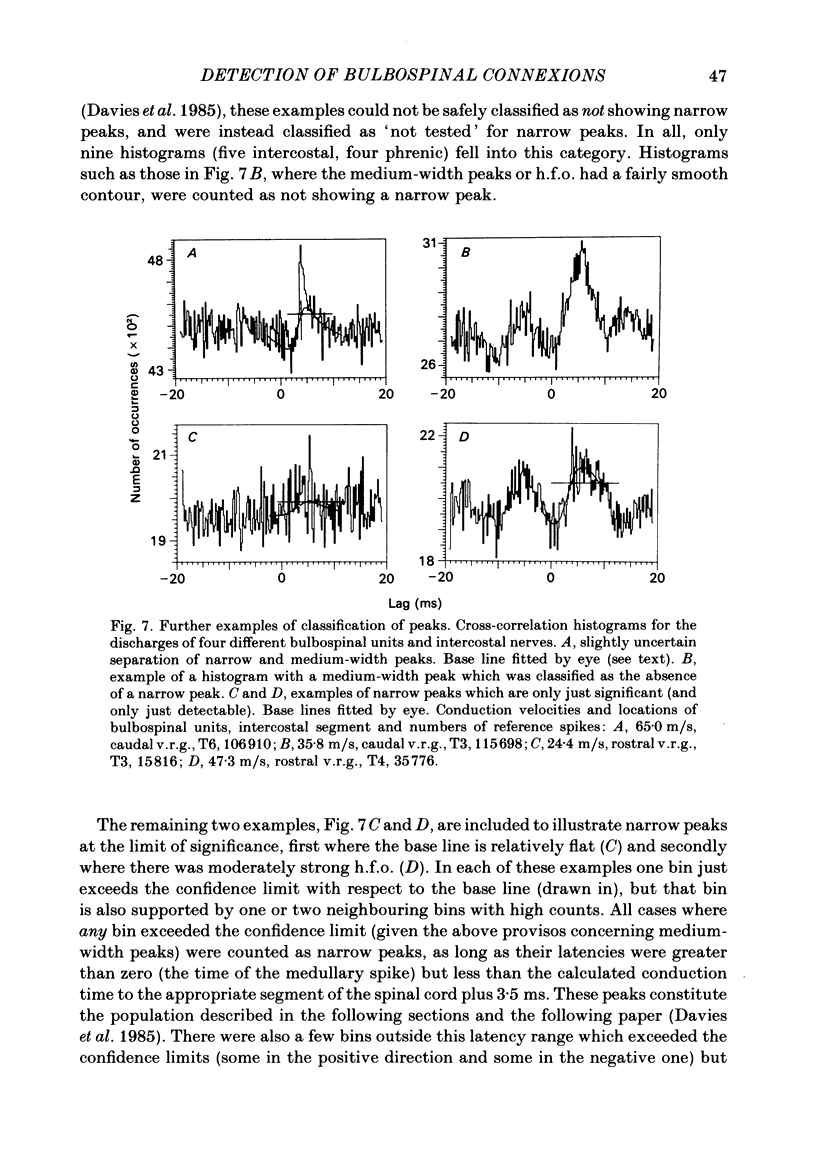
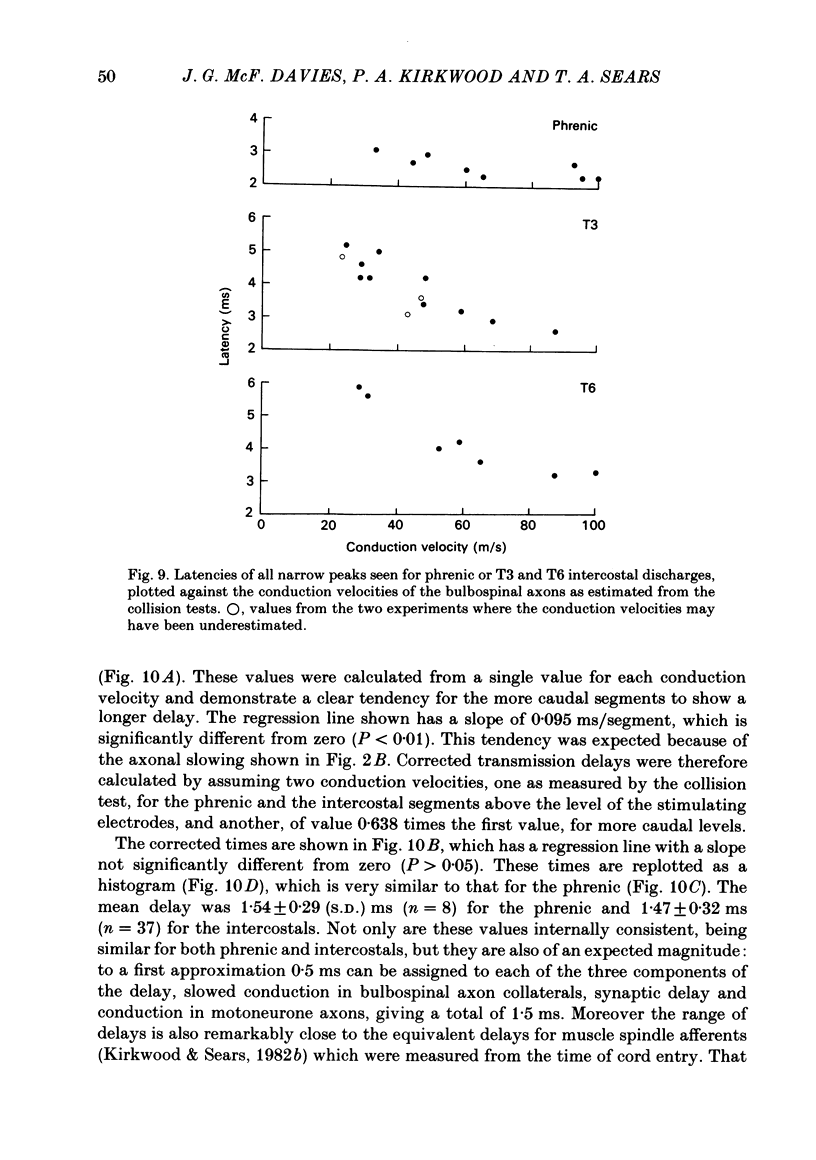
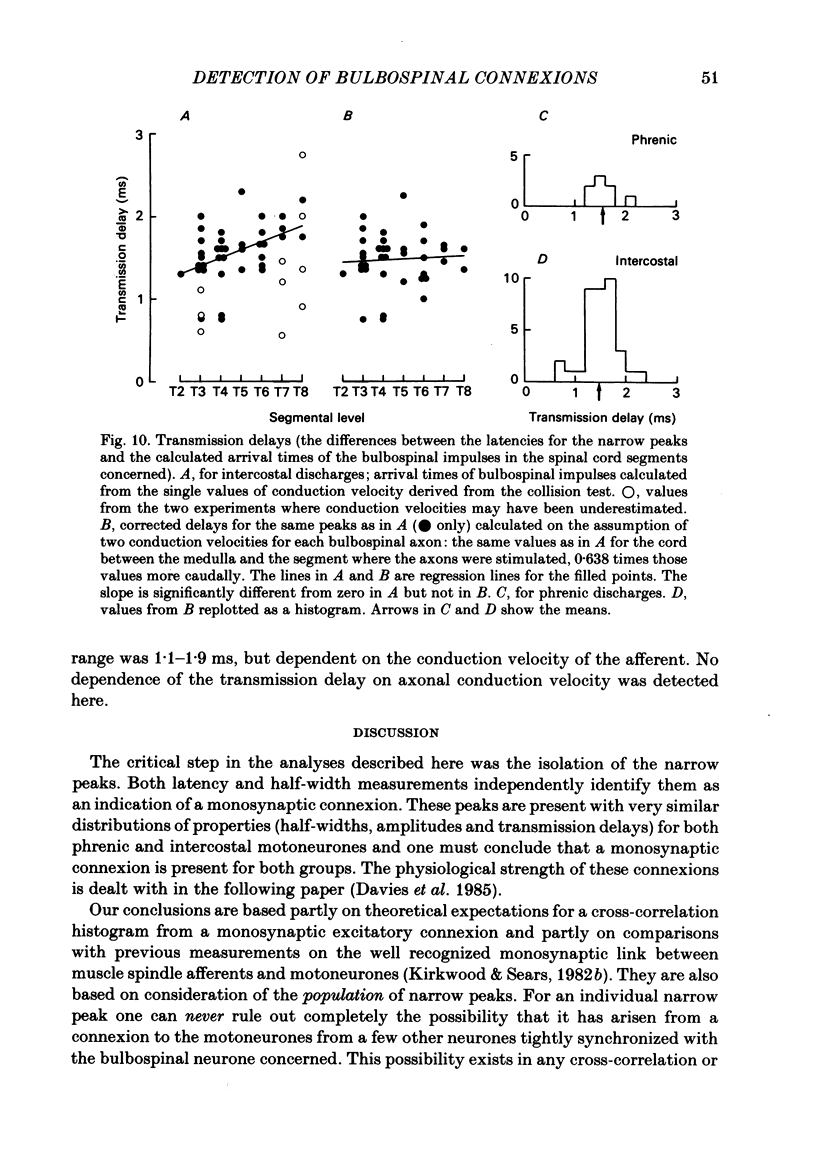
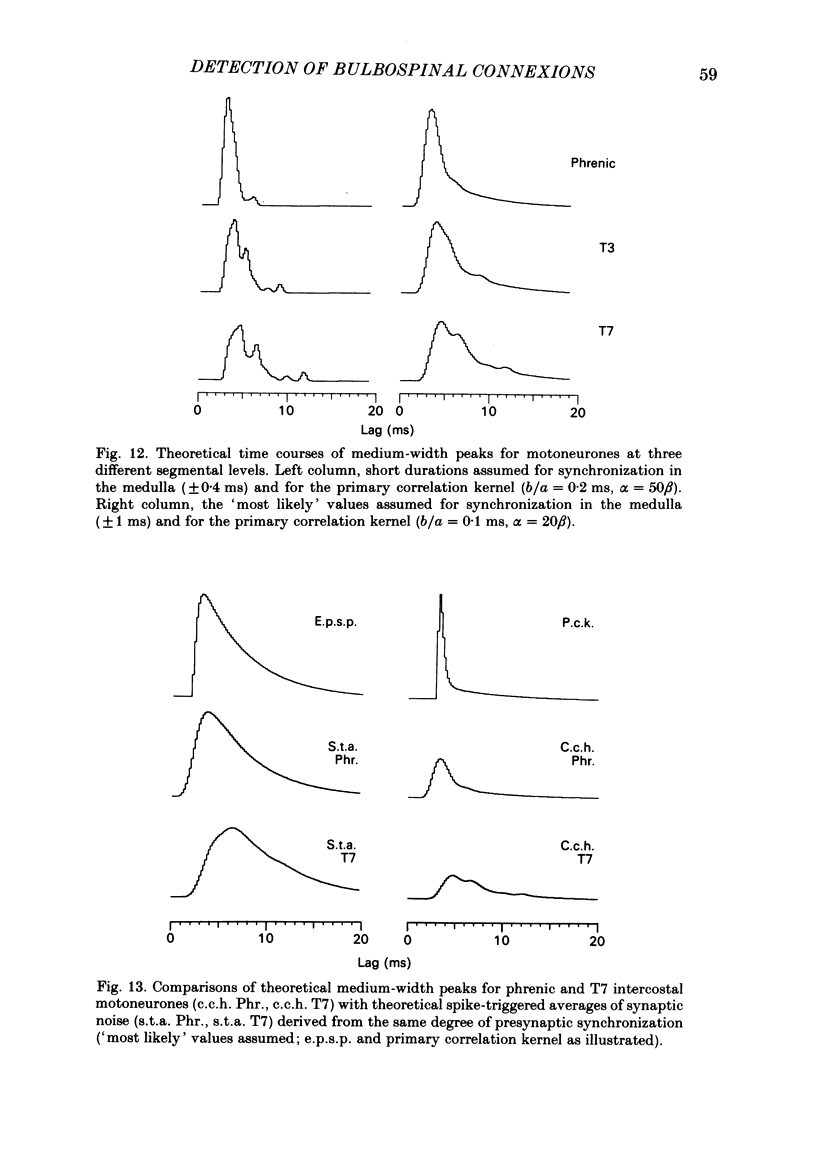
Abstract
Simultaneous recordings were made of the discharges of inspiratory bulbospinal neurones and phrenic or external intercostal alpha-motoneurones in the anaesthetized cat. The connexions between these neurones were studied by the construction of cross-correlation histograms from their discharges. Peaks observed in the cross-correlation histograms were divided into three groups on the basis of their time courses: narrow, medium-width and high-frequency oscillations (h.f.o.). Narrow peaks were defined as having half-widths less than 1.1 ms and medium-width peaks as having half-widths greater than this, while h.f.o. was characterized by periodic waves in the range 60-120 Hz. H.f.o. peaks were interpreted as being derived from the well known periodic synchronization of medullary inspiratory neurones in this frequency range. The time courses and latencies of the medium-width peaks could be quantitatively explained by a simple model representing excitation of the motoneurones by bulbospinal neurones whose discharges showed synchronization within +/- 1 ms of the reference spike, together with temporal dispersion in bulbospinal axons having a distribution of conduction velocities given by the measurements of this study. Such an explanation was essential for some of the medium-width peaks, whose latencies were short compared to the conduction times to the spinal cord for their own axons, but for other medium-width peaks oligosynaptic excitation of the motoneurones from the identified bulbospinal neurones was another possible explanation. The narrow peaks were of appropriate durations for monosynaptic connexions and were all at appropriate latencies (0.6-2.4 ms after the calculated arrival time of the bulbospinal impulse in the segment concerned). It is concluded from the observations of narrow peaks that monosynaptic excitation exists between inspiratory bulbospinal neurones and both phrenic and external intercostal motoneurones. However, because of the existence of presynaptic synchronization, as shown by the presence of the medium-width peaks, such a conclusion is predicated upon being able to discriminate against such an effect. The model showed that this restriction applies just as much to the measurements of excitatory post-synaptic potentials (e.p.s.p.s) by spike-triggered averaging as it does to cross-correlation measurements. We suggest that the discrimination against presynaptic synchronization here was possible only because the long conduction distance created temporal dispersion in the synchronized presynaptic impulses.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger A. J. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977 Oct 28;135(2):231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L. Localisation et étude des neurones respiratoires bulbaires. Mise en jeu antidromique par stimulation spinale ou vagale. J Physiol (Paris) 1971 Jan-Feb;63(1):5–40. [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Velluti J. C. Excitability of bulbar respiratory neurones: a study using microiontophoretic applications of depolarizing agents. Brain Res. 1980 Jun 9;191(2):359–377. doi: 10.1016/0006-8993(80)91287-1. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L. Discharge properties of dorsal medullary inspiratory neurons: relation to pulmonary afferent and phrenic efferent discharge. J Neurophysiol. 1984 Apr;51(4):753–776. doi: 10.1152/jn.1984.51.4.753. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Piercey M. F., Gootman P. M., Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Res. 1974 Dec 6;81(2):319–324. doi: 10.1016/0006-8993(74)90946-9. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Synchronization of discharge, spontaneous and evoked, between inspiratory neurons. Acta Neurobiol Exp (Wars) 1973;33(1):189–218. [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko L., Merrill E. G., Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983 Dec 30;43(2-3):285–291. doi: 10.1016/0304-3940(83)90202-1. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Sommer D., Cohen M. I. Short time scale correlations between discharges of medullary respiratory neurons. J Neurophysiol. 1980 May;43(5):1284–1295. doi: 10.1152/jn.1980.43.5.1284. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Speck D. F. Interactions among inspiratory neurons in dorsal and ventral respiratory groups in cat medulla. J Neurophysiol. 1983 Feb;49(2):472–490. doi: 10.1152/jn.1983.49.2.472. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller J. H., Schlag J. D. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976 Aug 13;112(2):283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Graham K., Duffin J. Cross-correlation of medullary dorsomedial inspiratory neurons in the cat. Exp Neurol. 1982 Mar;75(3):627–643. doi: 10.1016/0014-4886(82)90030-9. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm T. M., Reinking R. M., Roscoe D. D., Stuart D. G. Synchronous afferent discharge from a passive muscle of the cat: significance for interpreting spike-triggered averages. J Physiol. 1985 Aug;365:77–102. doi: 10.1113/jphysiol.1985.sp015760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Bianchi A. L. A cross-correlation study of interactions among respiratory neurons of dorsal, ventral and retrofacial groups in cat medulla. Brain Res. 1984 Jun 4;302(1):19–31. doi: 10.1016/0006-8993(84)91281-2. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R. Connexions entre les neurones inspiratoires bulbaires et les motoneurones phréniques et intercostaux. J Physiol (Paris) 1976;72(8):987–1000. [PubMed] [Google Scholar]
- Hilaire G., Monteau R. Facteurs déterminant l'order de recrutement des motoneurones phréniques. J Physiol (Paris) 1979;75(7):765–781. [PubMed] [Google Scholar]
- King G. W., Knox C. K. Types and locations of respiratory-related neurons in lateral tegmental field of cat medulla oblongata. Brain Res. 1984 Mar 19;295(2):301–315. doi: 10.1016/0006-8993(84)90979-x. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D., Westgaard R. H. The spatial distribution of synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:137–155. doi: 10.1113/jphysiol.1982.sp014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984 May;350:225–251. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter F., Richter D. W., Camerer H., Senekowitsch R. Morphological and electrical description of medullary respiratory neurons of the cat. Pflugers Arch. 1977 Nov 25;372(1):7–16. doi: 10.1007/BF00582200. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981 Jun;4(1):1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lipski J., Kubin L., Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 1983 Dec 12;288(1-2):105–118. doi: 10.1016/0006-8993(83)90085-9. [DOI] [PubMed] [Google Scholar]
- Lipski J., Trzebski A., Kubin L. Excitability changes of dorsal inspiratory neurons during lung inflations as studied by measurement of antidromic invasion latencies. Brain Res. 1979 Jan 26;161(1):25–38. doi: 10.1016/0006-8993(79)90193-8. [DOI] [PubMed] [Google Scholar]
- Madden K. P., Remmers J. E. Short time scale correlations between spike activity of neighboring respiratory neurons of nucleus tractus solitarius. J Neurophysiol. 1982 Sep;48(3):749–760. doi: 10.1152/jn.1982.48.3.749. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single central Ia afferent fibres projecting to motoneurones. J Physiol. 1979 Nov;296:315–327. doi: 10.1113/jphysiol.1979.sp013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S., Susswein A., Uchino Y., Wilson V. J. Synaptic actions of individual vestibular neurones on cat neck motoneurones. J Physiol. 1977 Nov;272(2):367–382. doi: 10.1113/jphysiol.1977.sp012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon B. R., Duffin J. Cross-correlation of medullary respiratory neurons in the cat. Exp Neurol. 1978 Aug;61(1):15–30. doi: 10.1016/0014-4886(78)90178-4. [DOI] [PubMed] [Google Scholar]


