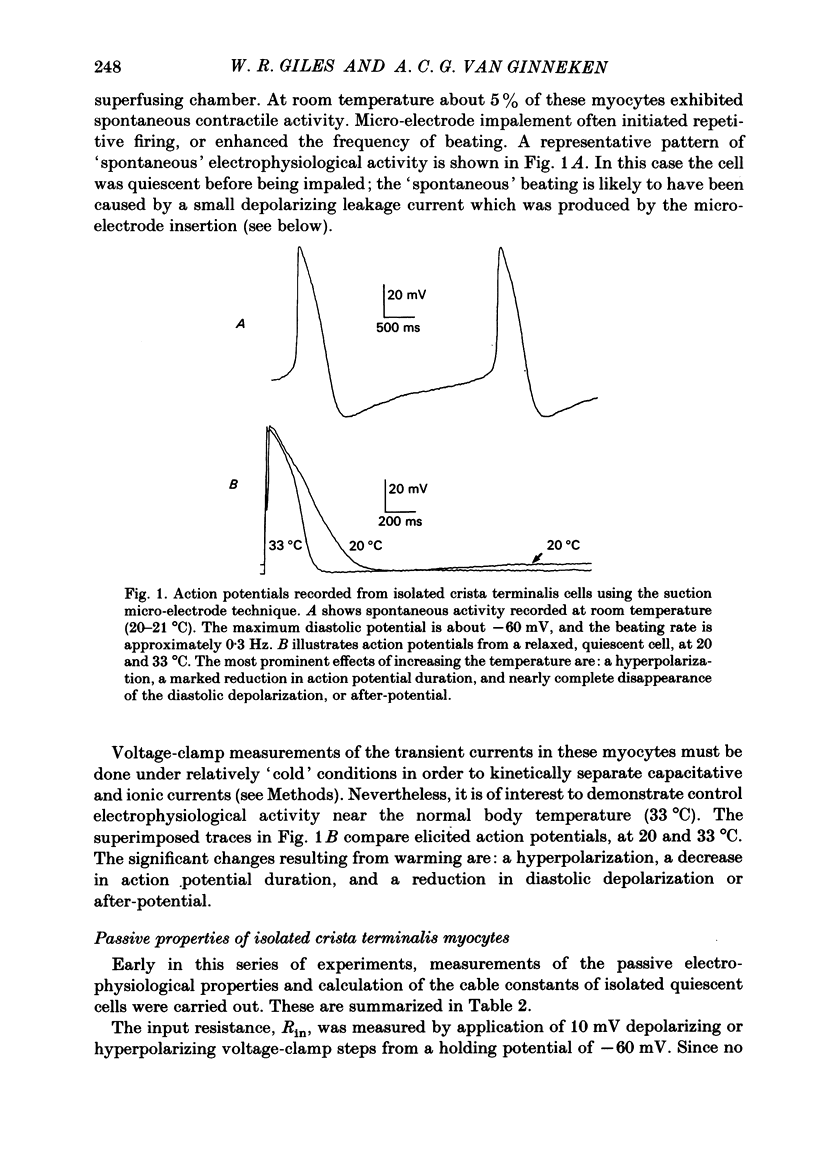
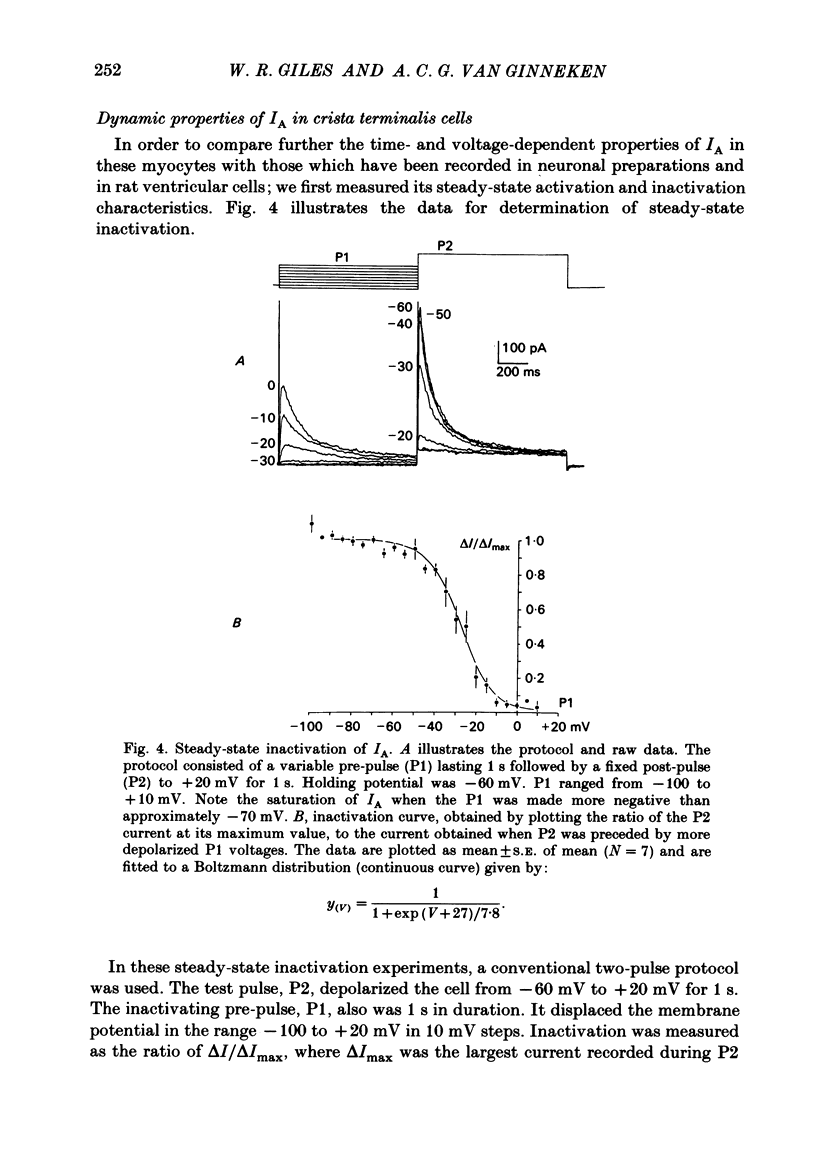
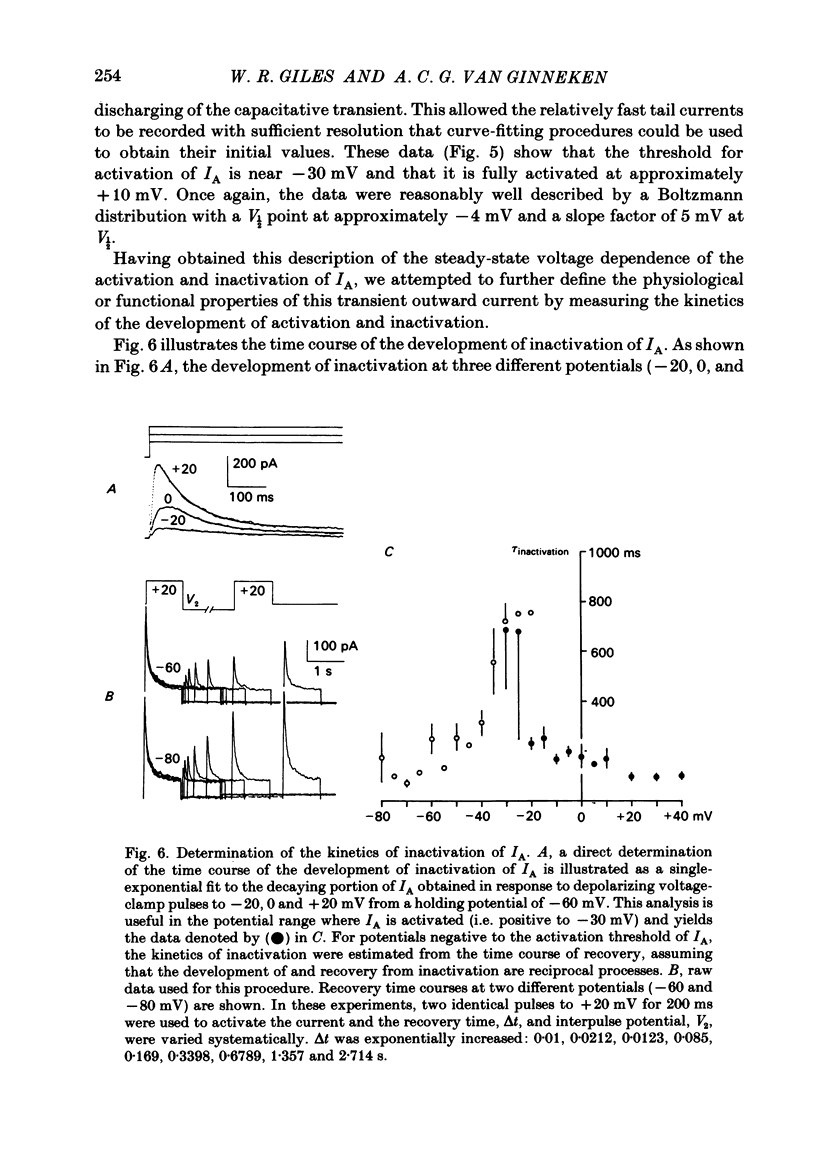
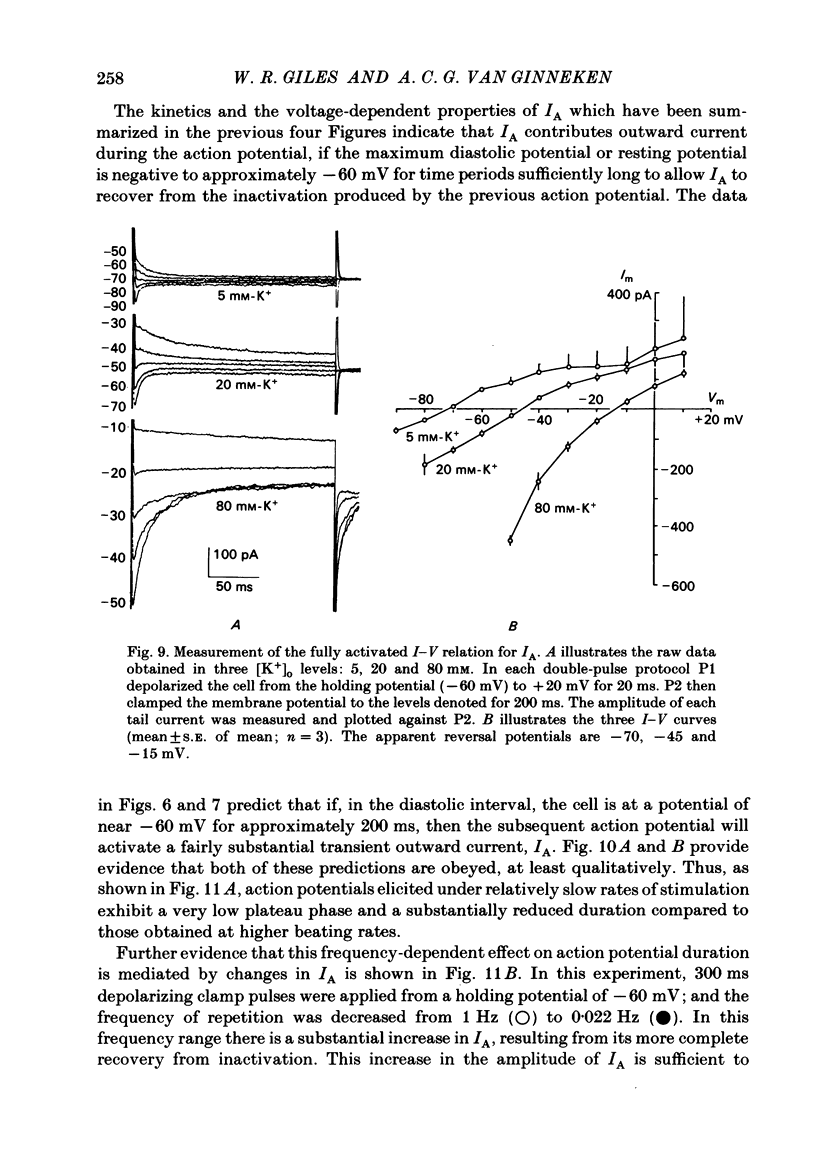
Abstract
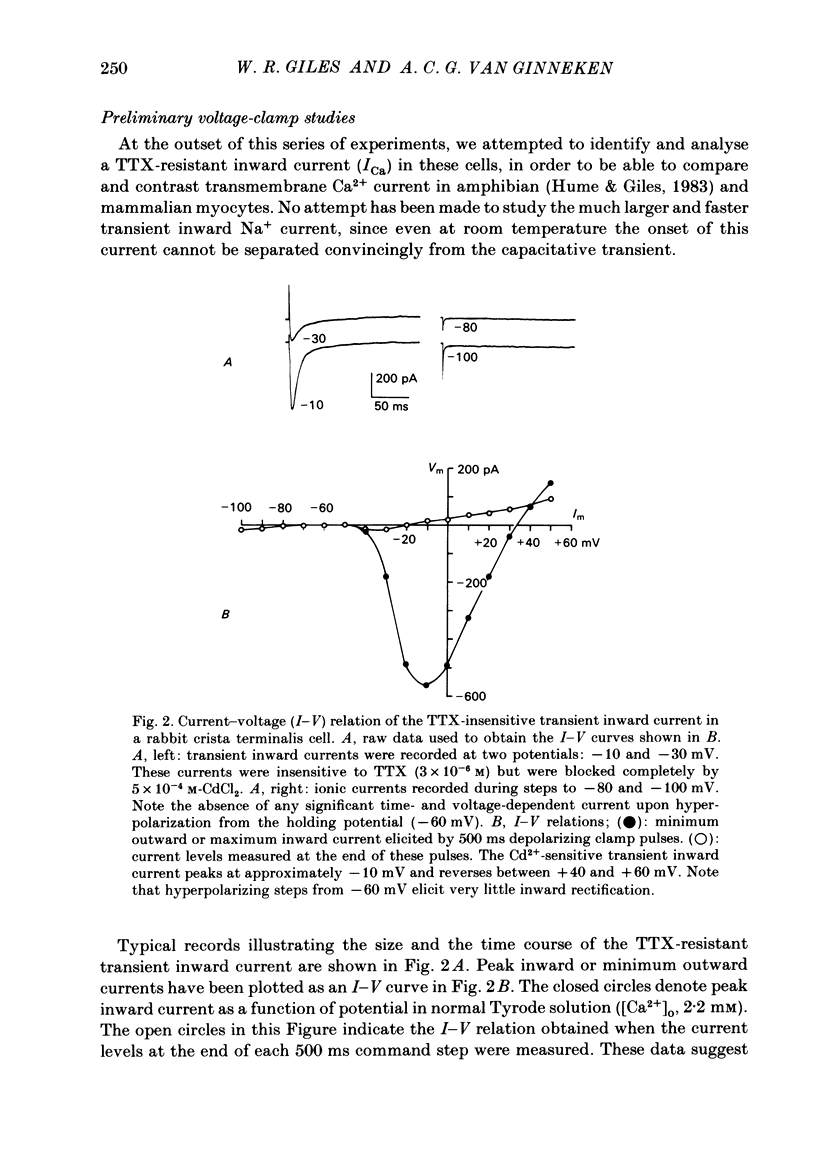
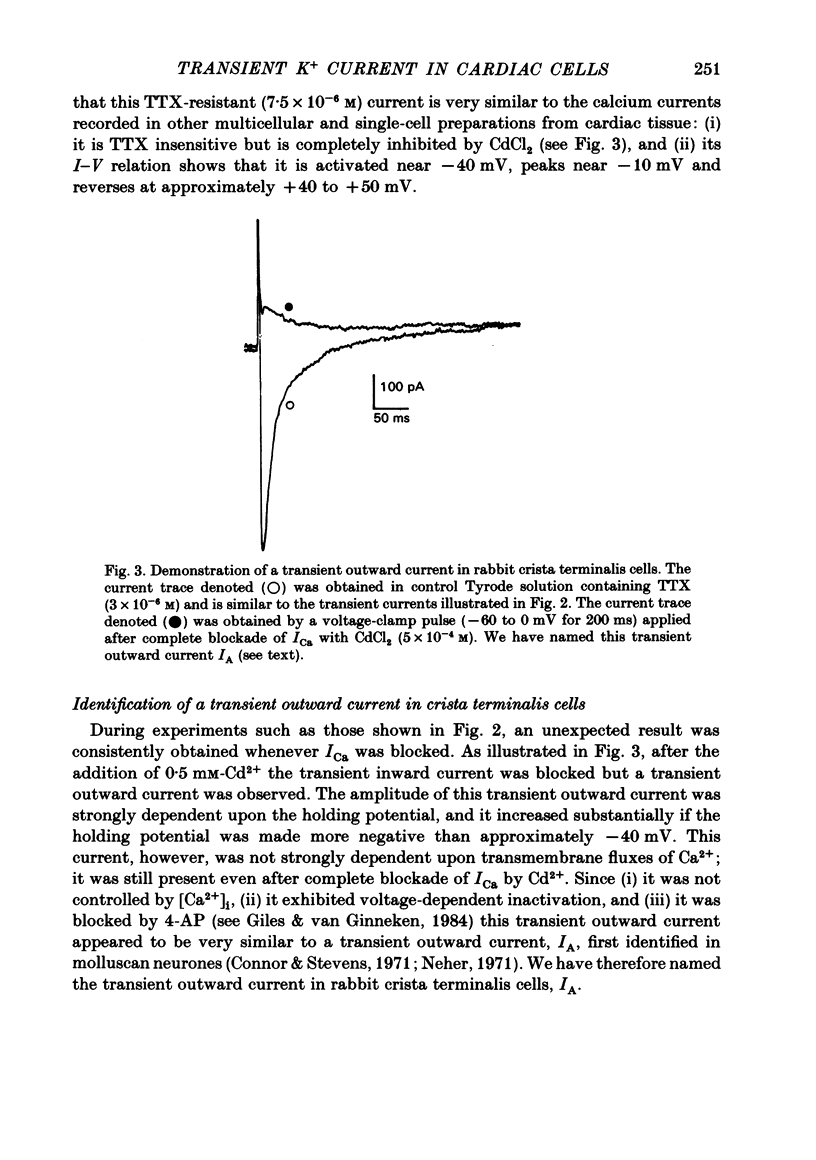
Voltage-clamp experiments were carried out with the objective of identifying and characterizing the time- and voltage-dependent properties of a transient outward current recorded in single myocytes from the crista terminalis region of the rabbit heart. A collagenase enzymic dispersion procedure similar to that described by Desilets & Horackova (1982) was used to obtain these viable individual myocytes. Transmembrane ionic currents were recorded using a single micro-electrode voltage-clamp technique. In experiments aimed at studying a tetrodotoxin-resistant transient inward current, (ICa); a transient outward current was consistently recorded following blockade of ICa with Cd2+ (5 X 10(-4) M). The time and voltage dependence of the activation and inactivation of this current were measured. Its steady-state inactivation curve spans the voltage range -70 to -10 mV, and it is activated between -20 and +10 mV. The reversal potential of this transient outward current is approximately -75 mV in [K+]O 5 mM, suggesting that it is carried mainly by K+. This transient outward current can be inhibited completely by external application of 4-aminopyridine (4-AP, 3 mM). The time- and voltage-dependent properties, the reversal potential, and the sensitivity to 4-AP of this transient outward current are all very similar to those of a transient outward current first identified in molluscan neurones. Hence, we have labelled it, IA. Selective inhibition of IA and knowledge of its voltage- and time-dependent properties yield specific predictions concerning its role in the action potential of isolated crista terminalis cells. Consistent with these predictions, a decrease in stimulus rate is found to decrease the duration of the action potential and vice versa; and application of effective doses of 4-AP results in a substantial lengthening of the action potential. These results are discussed in terms of the possible physiological role of IA in subsidiary or follower pace-maker tissue, and the anatomical and physiological heterogeneity of the sino-atrial node region of the rabbit heart.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Allen D. G. On the relationship between action potential duration and tension in cat papillary muscle. Cardiovasc Res. 1977 May;11(3):210–218. doi: 10.1093/cvr/11.3.210. [DOI] [PubMed] [Google Scholar]
- Anderson T. W., Johnson E. A. The repolarization phase of the cardiac action potential: a comparative study of rate-induced changes in its waveform. J Mol Cell Cardiol. 1976 Feb;8(2):103–121. doi: 10.1016/0022-2828(76)90024-9. [DOI] [PubMed] [Google Scholar]
- Bleeker W. K., Mackaay A. J., Masson-Pévet M., Bouman L. N., Becker A. E. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980 Jan;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Brown H. F. Electrophysiology of the sinoatrial node. Physiol Rev. 1982 Apr;62(2):505–530. doi: 10.1152/physrev.1982.62.2.505. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Repolarisation and frequency in cardiac cells. J Physiol (Paris) 1977;73(7):903–923. [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilets M., Horackova M. Na+-dependence of 45Ca2+ uptake in adult rat isolated cardiac cells. Biochim Biophys Acta. 1982 Oct 11;721(2):144–157. doi: 10.1016/0167-4889(82)90062-3. [DOI] [PubMed] [Google Scholar]
- Dow J. W., Harding N. G., Powell T. Isolated cardiac myocytes. I. Preparation of adult myocytes and their homology with the intact tissue. Cardiovasc Res. 1981 Sep;15(9):483–514. doi: 10.1093/cvr/15.9.483. [DOI] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The dynamic chloride component of membrane current in Purkinje fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(3):197–212. doi: 10.1007/BF01844100. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Hiraoka M. The positive dynamic current and its inactivation properties in cardiac Purkinje fibres. J Physiol. 1973 Nov;234(3):569–586. doi: 10.1113/jphysiol.1973.sp010361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. O., Strauss H. C. Intracellular potassium activity in rabbit sinoatrial node. Evaluation during spontaneous activity and arrest. Circ Res. 1982 Sep;51(3):271–279. doi: 10.1161/01.res.51.3.271. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Active and passive electrical properties of single bullfrog atrial cells. J Gen Physiol. 1981 Jul;78(1):19–42. doi: 10.1085/jgp.78.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978 Apr;58(2):461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Irisawa H. Electrophysiology of single cardiac cells. Jpn J Physiol. 1984;34(3):375–388. doi: 10.2170/jjphysiol.34.375. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Influence of chloride, potassium, and tetraethylammonium on the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):117–138. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukushkin N. I., Gainullin R. Z., Sosunov E. A. Transient outward current and rate dependence of action potential duration in rabbit cardiac ventricular muscle. Pflugers Arch. 1983 Oct;399(2):87–92. doi: 10.1007/BF00663902. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson-Pévet M., Gros D., Besselsen E. The caveolae in rabbit sinus node and atrium. Cell Tissue Res. 1980;208(2):183–196. doi: 10.1007/BF00234869. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Strontium, nifedipine and 4-aminopyridine modify the time course of the action potential in cells from rat ventricular muscle. Br J Pharmacol. 1984 Mar;81(3):551–556. doi: 10.1111/j.1476-5381.1984.tb10108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Nakayama T., Kurachi Y., Irisawa H. Resting K conductances in pacemaker and non-pacemaker heart cells of the rabbit. Jpn J Physiol. 1984;34(2):245–254. doi: 10.2170/jjphysiol.34.245. [DOI] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon M. E., Safronova V. G. The rest-dependent depression of action potential duration in rabbit myocardium and the possible role of the transient outward current. A pharmacological analysis. J Physiol (Paris) 1982;78(5):461–466. [PubMed] [Google Scholar]
- Segal M., Rogawski M. A., Barker J. L. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984 Feb;4(2):604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M. Electrogenesis of the plateau and pacemaker potential. Annu Rev Physiol. 1979;41:425–440. doi: 10.1146/annurev.ph.41.030179.002233. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]