Abstract
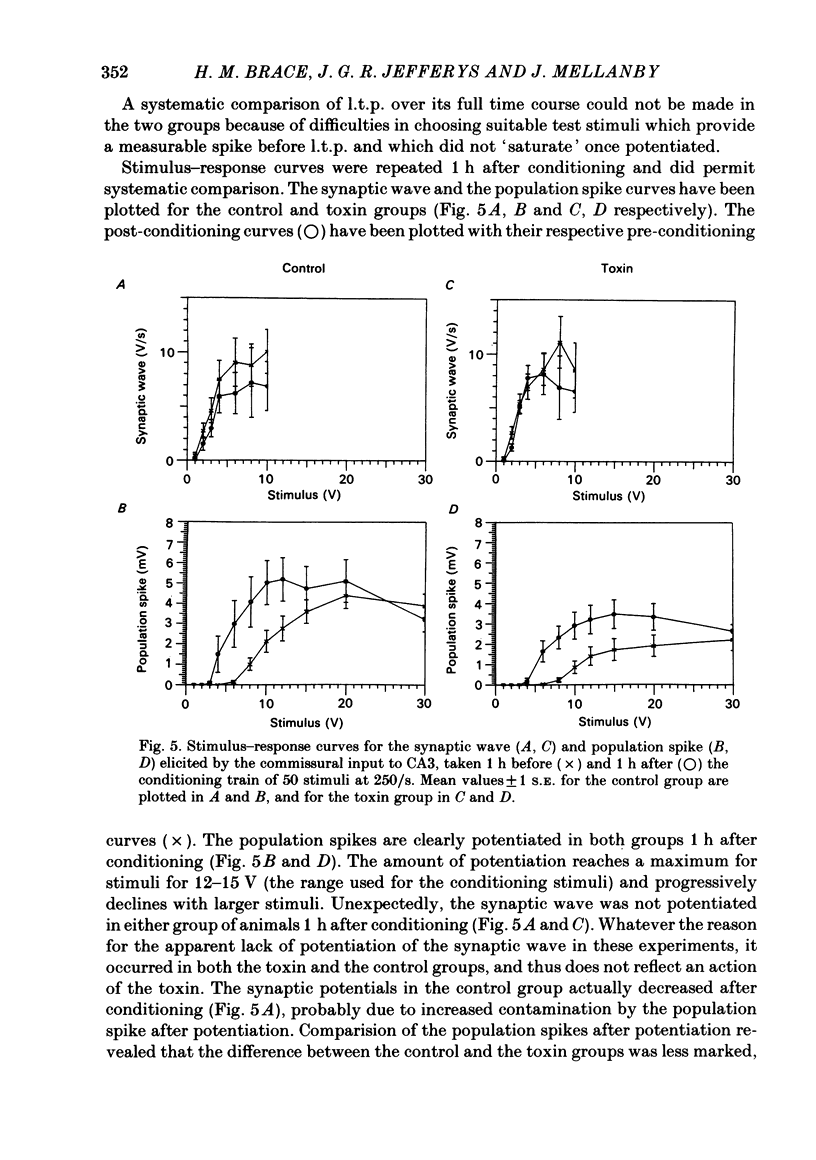
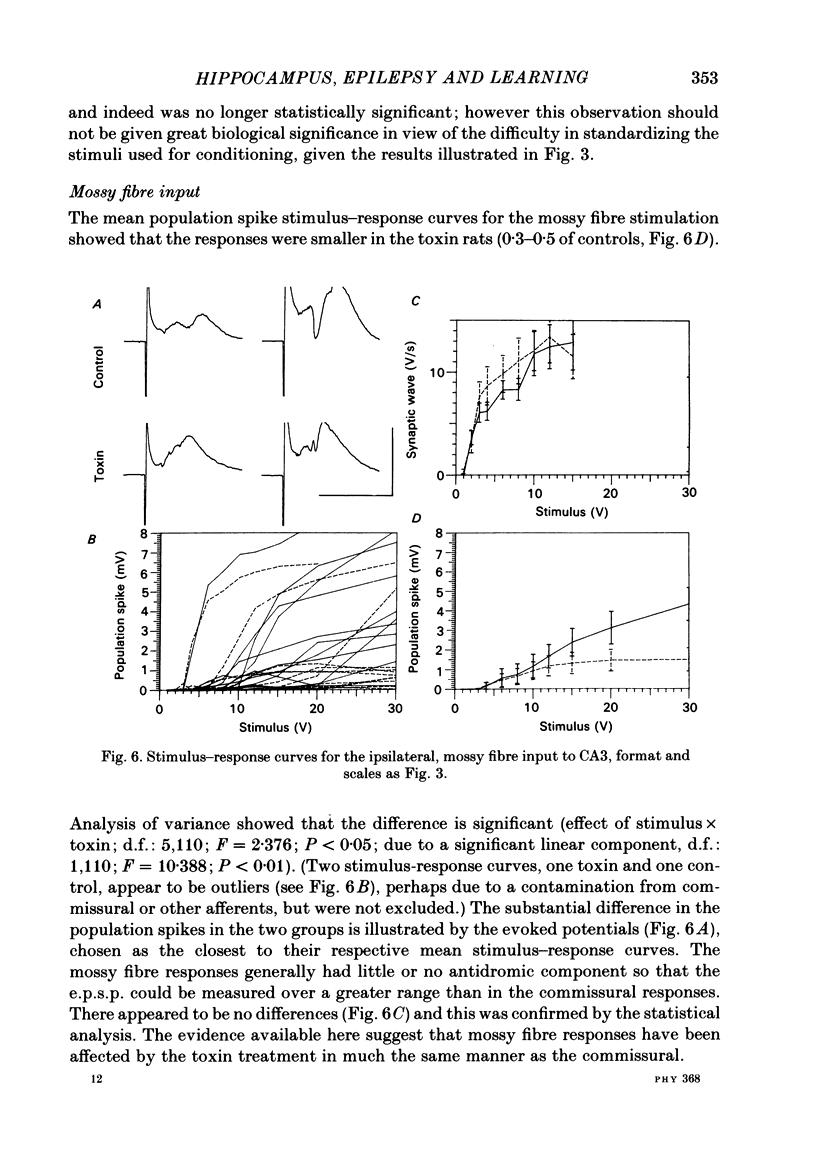
A chronic epileptic syndrome can be induced by injecting minute doses of tetanus toxin into rat hippocampi. This causes intermittent epileptic fits over a period of 2-4 weeks, after which the fits cease, and the electroencephalogram (e.e.g.) appears to return to normal over the following 2-3 weeks. However, once they have recovered from the seizures, the rats exhibit a remarkably persistent impairment of learning and memory, which is the subject of the present study. Learning ability was assessed using a radial arm maze task, in which the rats had to visit each of eight arms for a food reward. The toxin-injected rats learnt this task more slowly than control-injected. Evoked potentials from the CA3 pyramidal cells were recorded in terminal experiments under halothane anaesthesia. Long term potentiation of the post-synaptic response to the commissural pathway from the contralateral hippocampus appeared to be unaffected by the previous toxin treatment, at least over periods of up to 5 h. The toxin-injected group differed from the control in having consistently smaller post-synaptic population spikes in their evoked responses, so that stimuli were less effective in exciting the post-synaptic neurones. This applied both to the contralateral commissural input, and to the ipsilateral mossy fibre input. No differences were found between the toxin and control groups in the size of the antidromic population spike in the commissural response, or in the population excitatory post-synaptic potential (e.p.s.p.) for either input. Thus the depressed output from CA3 pyramidal cells cannot be explained either by a loss of these neurones (confirming earlier neuropathological observations), or by a loss of excitatory afferents. While its precise cause remains unknown, the depressed output from the CA3 region was statistically correlated with the learning impairment, and we believe provides a reasonable explanation of this behavioural deficit.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Sundberg S. H., Sveen O., Swann J. W., Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980 May;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A., McNaughton B. L. Excitability changes in hippocampal granule cells of senescent rat. Prog Brain Res. 1983;58:445–451. doi: 10.1016/S0079-6123(08)60047-3. [DOI] [PubMed] [Google Scholar]
- Barnes C. A., McNaughton B. L. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980 Dec;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979 Feb;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Davies J. Reversible effects of low doses of tetanus toxin on synaptic inhibition in the substantia nigra and turning behaviour in the rat. Brain Res. 1980 Mar 10;185(2):455–459. doi: 10.1016/0006-8993(80)91086-0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Thompson P. A., Davies J., Mellanby J. In vitro effect of tetanus toxin on GABA release form rat hippocampal slices. J Neurochem. 1981 Oct;37(4):1039–1041. doi: 10.1111/j.1471-4159.1981.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. The effects of tetanus toxin on the motor end-plates of the mouse. An electron microscopic study. J Neurol Sci. 1973 Jun;19(2):153–167. doi: 10.1016/0022-510x(73)90159-7. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George G., Mellanby J. Memory deficits in an experimental hippocampal epileptiform syndrome in rats. Exp Neurol. 1982 Mar;75(3):678–689. doi: 10.1016/0014-4886(82)90034-6. [DOI] [PubMed] [Google Scholar]
- Hawkins C. A., Mellanby J., Brown J. Antiepileptic and antiamnesic effect of carbamazepine in experimental limbic epilepsy. J Neurol Neurosurg Psychiatry. 1985 May;48(5):459–468. doi: 10.1136/jnnp.48.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K., Takano K. Effect of tetanus toxin on the excitatory and the inhibitory post-synaptic potentials in the cat motoneurone. J Physiol. 1983 Feb;335:319–333. doi: 10.1113/jphysiol.1983.sp014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Mellanby J., George G., Robinson A., Thompson P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):404–414. doi: 10.1136/jnnp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., George G. Tetanus toxin and experimental epilepsy in rats. Adv Cytopharmacol. 1979;3:401–408. [PubMed] [Google Scholar]
- Mellanby J., Hawkins C., Wilks L. The relationship between seizures and amnesia in experimental epilepsy. Acta Neurol Scand Suppl. 1984;99:119–124. doi: 10.1111/j.1600-0404.1984.tb05677.x. [DOI] [PubMed] [Google Scholar]
- Mellanby J., Mellanby H., Pope D., Van Heyningen W. E. Ganglioside as a prophylactic agent in experimental tetanus in mice. J Gen Microbiol. 1968 Dec;54(2):161–168. doi: 10.1099/00221287-54-2-161. [DOI] [PubMed] [Google Scholar]
- Mellanby J., Renshaw M., Cracknell H., Rands G., Thompson P. Long-term impairment of learning ability in rats after an experimental hippocampal epileptiform syndrome. Exp Neurol. 1982 Mar;75(3):690–699. doi: 10.1016/0014-4886(82)90035-8. [DOI] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W., Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Tuff L. P., Racine R. J., Adamec R. The effects of kindling on GABA-mediated inhibition in the dentate gyrus of the rat. I. Paired-pulse depression. Brain Res. 1983 Oct 24;277(1):79–90. doi: 10.1016/0006-8993(83)90909-5. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Comparative aspects of studies of amnesia. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 25;298(1089):97–109. doi: 10.1098/rstb.1982.0075. [DOI] [PubMed] [Google Scholar]


