Abstract
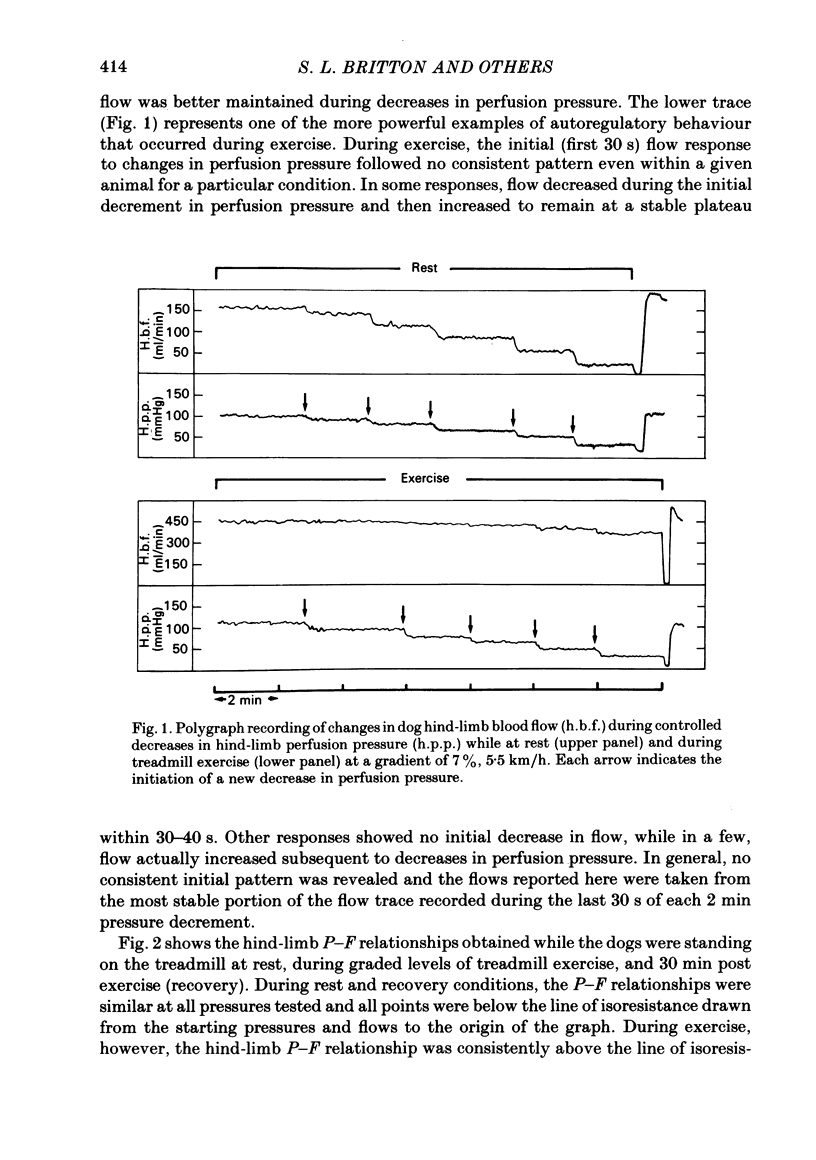
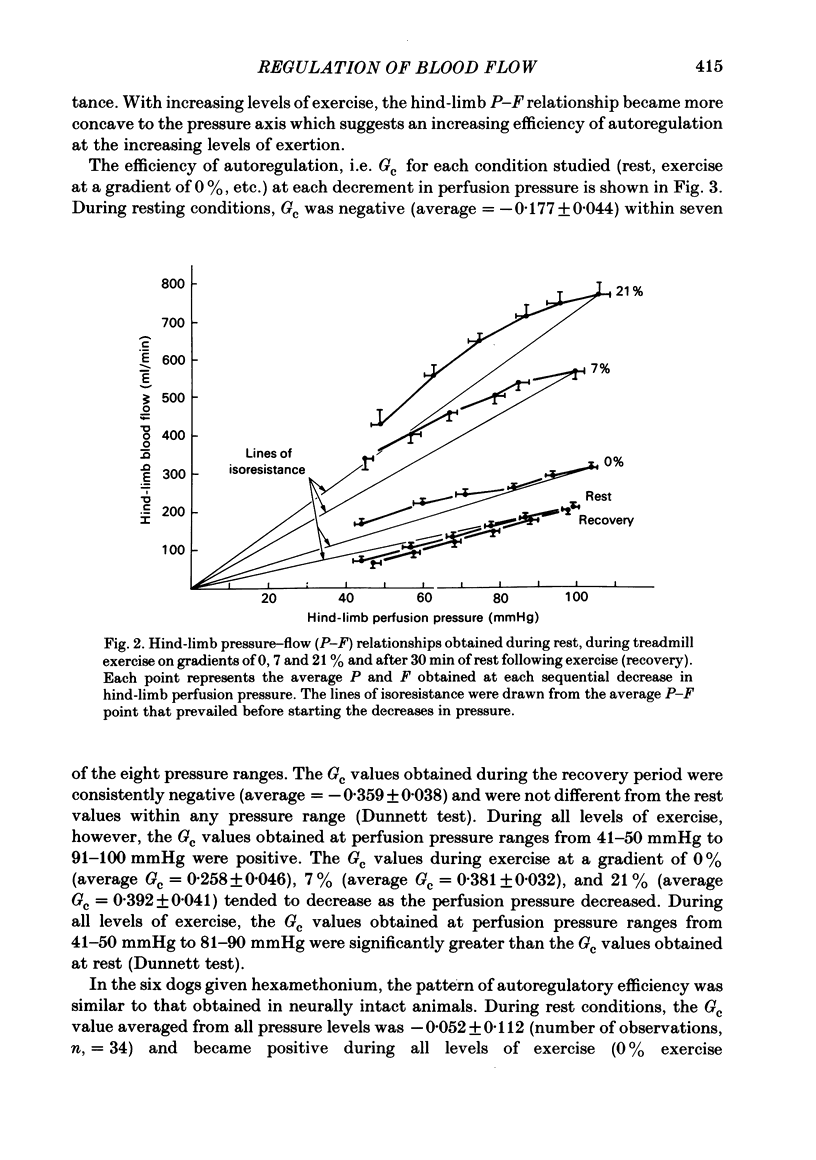
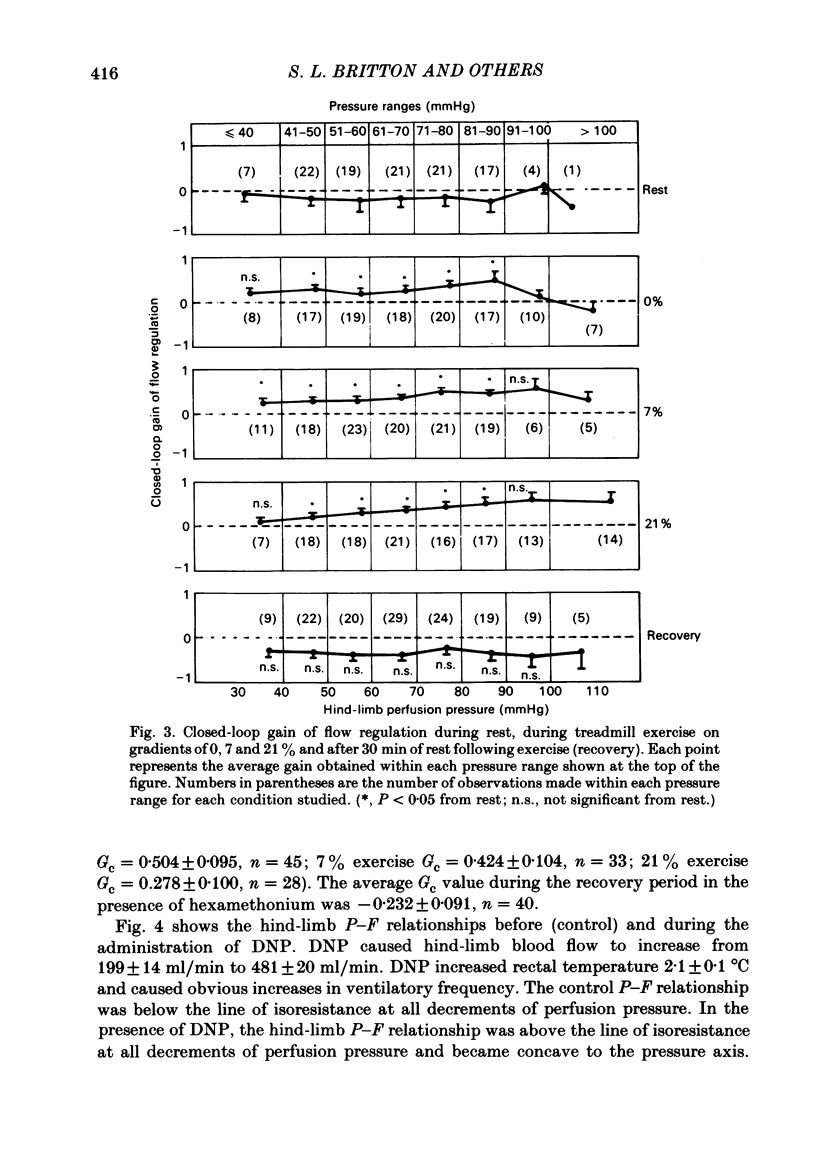
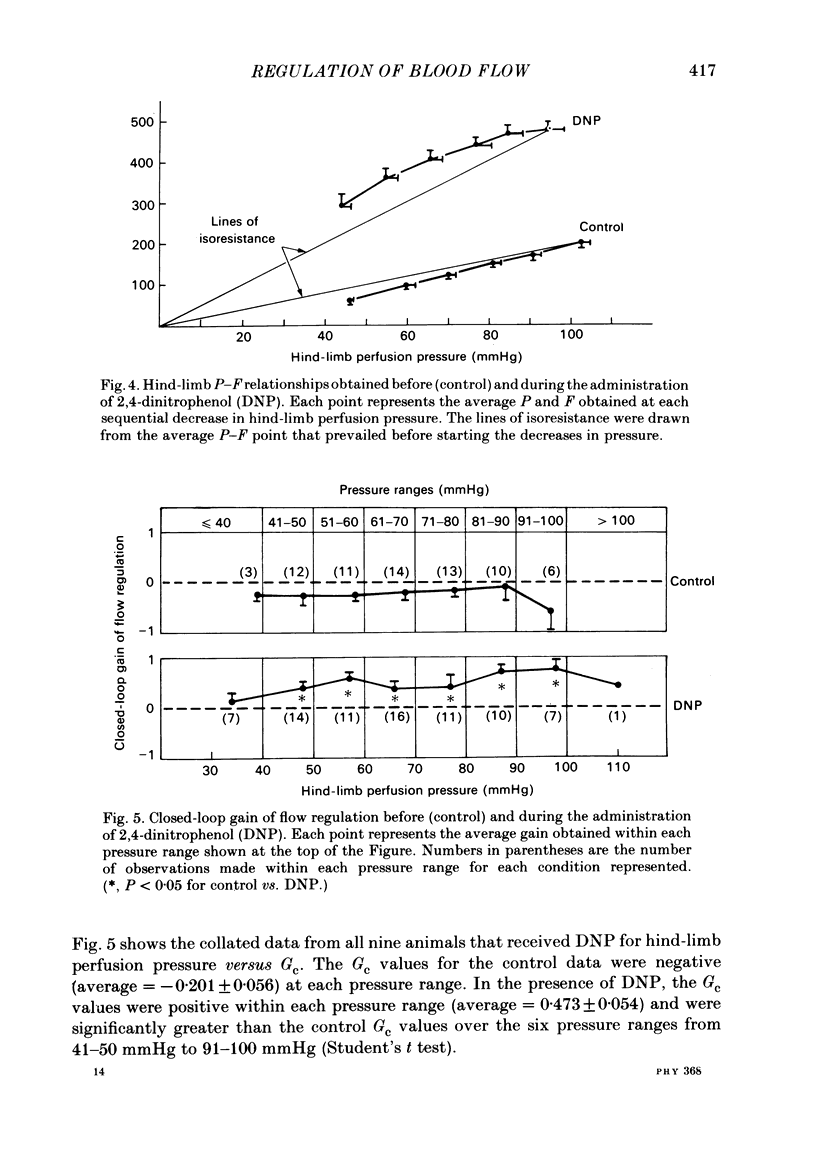
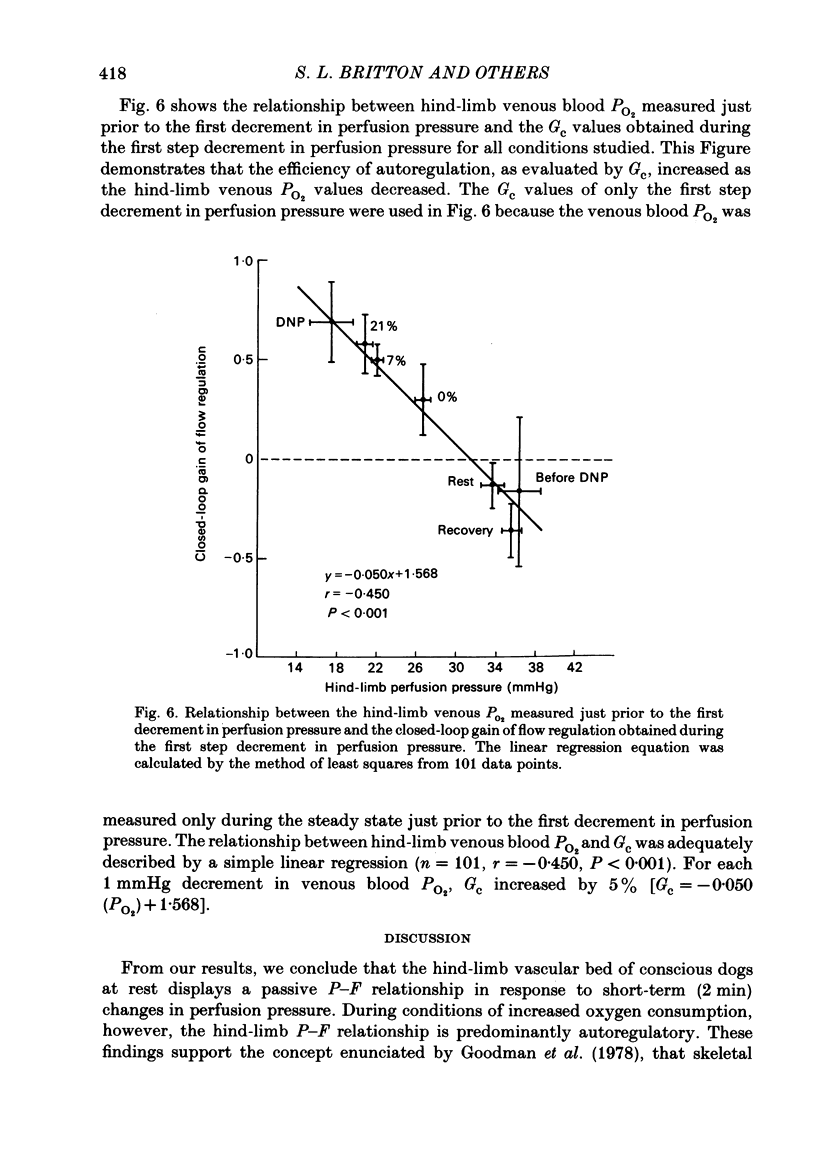
We evaluated the efficiency of blood flow autoregulation of the hind-limb vascular bed of eleven conscious dogs during: resting conditions; graded levels of treadmill exercise; and increases in oxygen consumption produced by the administration of 2,4-dinitrophenol (DNP). Blood flow to the left hind limb was measured with an electromagnetic flow probe on the left external iliac artery. Hind-limb perfusion pressure was measured from a catheter in the deep femoral artery and was controlled via an externally inflatable occlusion cuff positioned just distal to the flow probe. Arterial pressure was measured in the abdominal aorta. Experiments were performed 5-16 days after instrumentation. Hind-limb pressure-flow (P-F) relationships were evaluated by decreasing hind-limb perfusion pressure in 4-5 small sequential 'square-wave' steps of 10-15 mmHg each while measuring flow. Each step decrease in perfusion pressure was maintained for 2 min. The efficiency of autoregulation was quantified by calculating the closed-loop gain of flow regulation (Gc) at each decrement in perfusion pressure utilizing the equation: Gc = 1-[(F0-Fn/F0)/(P0-Pn/P0)] where F0 and P0 are the starting (control) flows and pressures prevailing prior to decreasing perfusion pressure, and Fn and Pn are the new flows and pressures at each decrement in perfusion pressure. A Gc value less than 0 indicates a predominantly passive P-F relationship, while a Gc of 1 is perfect autoregulation of flow. When the dogs were at rest, decrements in hind-limb perfusion pressure were accompanied by almost equivalent decreases in flow, i.e. no autoregulation occurred, and Gc averaged -0.177 +/- 0.044 over the pressure range from 100-40 mmHg. During all levels of treadmill exercise (on gradients of 0, 7, or 21%), however, positive Gc values were found that averaged from 0.258 +/- 0.046 at a gradient of 0% to 0.392 +/- 0.041 at a gradient of 21% and were significantly different from Gc values found during rest at perfusion pressure ranges from 90-40 mmHg. The administration of DNP directly into the hind-limb circulation increased hind-limb blood flow from 199 to 481 ml/min. In the presence of DNP, Gc values were positive over perfusion pressure ranges from 100-40 mmHg and averaged 0.473 +/- 0.054. These data demonstrate that hind-limb blood flow is not autoregulated in resting dogs, but that significant autoregulation is manifest during conditions that increase oxygen consumption.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Altura B. T., Carella A., Turlapaty P. D., Weinberg J. Vascular smooth muscle and general anesthetics. Fed Proc. 1980 Apr;39(5):1584–1591. [PubMed] [Google Scholar]
- Arendshorst W. J. Autoregulation of renal blood flow in spontaneously hypertensive rats. Circ Res. 1979 Mar;44(3):344–349. doi: 10.1161/01.res.44.3.344. [DOI] [PubMed] [Google Scholar]
- BOWMAN W. C. The effect of muscle contraction on the blood flow and on the vascular responses to adrenaline, noradrenaline and isoprenaline in individual skeletal muscles of the cat. J Pharm Pharmacol. 1959 Nov;11:641–649. doi: 10.1111/j.2042-7158.1959.tb12608.x. [DOI] [PubMed] [Google Scholar]
- BURTON A. C., STINSON R. H. The measurement of tension in vascular smooth muscle. J Physiol. 1960 Sep;153:290–305. doi: 10.1113/jphysiol.1960.sp006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S. Bayliss response in the microcirculation. Fed Proc. 1968 Nov-Dec;27(6):1410–1415. [PubMed] [Google Scholar]
- Bayliss W. M. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902 May 28;28(3):220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Burn J. H. Blood flow during muscle contraction and the orbeli phenomenon in the dog. J Physiol. 1939 Feb 14;95(1):203–225. doi: 10.1113/jphysiol.1939.sp003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T. G., Samar R. E., Murphy W. R. Autoregulation versus other vasoconstrictors in hypertension. A critical review. Hypertension. 1979 May-Jun;1(3):324–330. doi: 10.1161/01.hyp.1.3.324. [DOI] [PubMed] [Google Scholar]
- Drake-Holland A. J., Laird J. D., Noble M. I., Spaan J. A., Vergroesen I. Oxygen and coronary vascular resistance during autoregulation and metabolic vasodilation in the dog. J Physiol. 1984 Mar;348:285–299. doi: 10.1113/jphysiol.1984.sp015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber J. E., Harris P. D., Wiegman D. L. Anesthetic depression of microcirculation, central hemodynamics, and respiration in decerebrate rats. Am J Physiol. 1982 Dec;243(6):H837–H843. doi: 10.1152/ajpheart.1982.243.6.H837. [DOI] [PubMed] [Google Scholar]
- Goodman A. H., Einstein R., Granger H. J. Effect of changing metabolic rate on local blood flow control in the canine hindlimb. Circ Res. 1978 Nov;43(5):769–776. doi: 10.1161/01.res.43.5.769. [DOI] [PubMed] [Google Scholar]
- Granger H. J., Goodman A. H., Cook B. H. Metabolic models of microcirculatory regulation. Fed Proc. 1975 Oct;34(11):2025–2030. [PubMed] [Google Scholar]
- Granger H. J., Goodman A. H., Granger D. N. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res. 1976 May;38(5):379–385. doi: 10.1161/01.res.38.5.379. [DOI] [PubMed] [Google Scholar]
- Granger H. J., Guyton A. C. Autoregulation of the total systemic circulation following destruction of the central nervous system in the dog. Circ Res. 1969 Oct;25(4):379–388. doi: 10.1161/01.res.25.4.379. [DOI] [PubMed] [Google Scholar]
- Henriksen O., Nielsen S. L., Paaske W. P., Sejrsen P. Autoregulation of blood flow in human cutaneous tissue. Acta Physiol Scand. 1973 Dec;89(4):538–543. doi: 10.1111/j.1748-1716.1973.tb05547.x. [DOI] [PubMed] [Google Scholar]
- JONES R. D., BERNE R. M. INTRINSIC REGULATION OF SKELETAL MUSCLE BLOOD FLOW. Circ Res. 1964 Feb;14:126–138. doi: 10.1161/01.res.14.2.126. [DOI] [PubMed] [Google Scholar]
- Johnson P. C., Henrich H. A. Metabolic and myogenic factors in local regulation of the microcirculation. Fed Proc. 1975 Oct;34(11):2020–2024. [PubMed] [Google Scholar]
- Johnson P. C., Wayland H. Regulation of blood flow in single capillaries. Am J Physiol. 1967 Jun;212(6):1405–1415. doi: 10.1152/ajplegacy.1967.212.6.1405. [DOI] [PubMed] [Google Scholar]
- KLERMAN G. L., COLE J. O. EFFECTS OF ANESTHESIA ON METABOLISM AND CELLULAR FUNCTIONS. A WORKSHOP HELD UNDER THE COMMITTEE ON ANESTHESIA OF THE NATIONAL ACADEMY OF SCIENCES--NATIONAL RESEARCH COUNCIL. Pharmacol Rev. 1965 Jun;17:183–263. [PubMed] [Google Scholar]
- Morff R. J., Granger H. J. Autoregulation of blood flow within individual arterioles in the rat cremaster muscle. Circ Res. 1982 Jul;51(1):43–55. doi: 10.1161/01.res.51.1.43. [DOI] [PubMed] [Google Scholar]
- REMENSNYDER J. P., MITCHELL J. H., SARNOFF S. J. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962 Sep;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Roy C. S., Brown J. G. The Blood-Pressure and its Variations in the Arterioles, Capillaries and Smaller Veins. J Physiol. 1880 Jul;2(5-6):323–446.1. doi: 10.1113/jphysiol.1880.sp000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAINSBY W. N. Autoregulation of blood flow in skeletal muscle during increased metabolic activity. Am J Physiol. 1962 Feb;202:273–276. doi: 10.1152/ajplegacy.1962.202.2.273. [DOI] [PubMed] [Google Scholar]
- Wyss C. R., Ardell J. L., Scher A. M., Rowell L. B. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol. 1983 Sep;245(3):H481–H486. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]


