Abstract
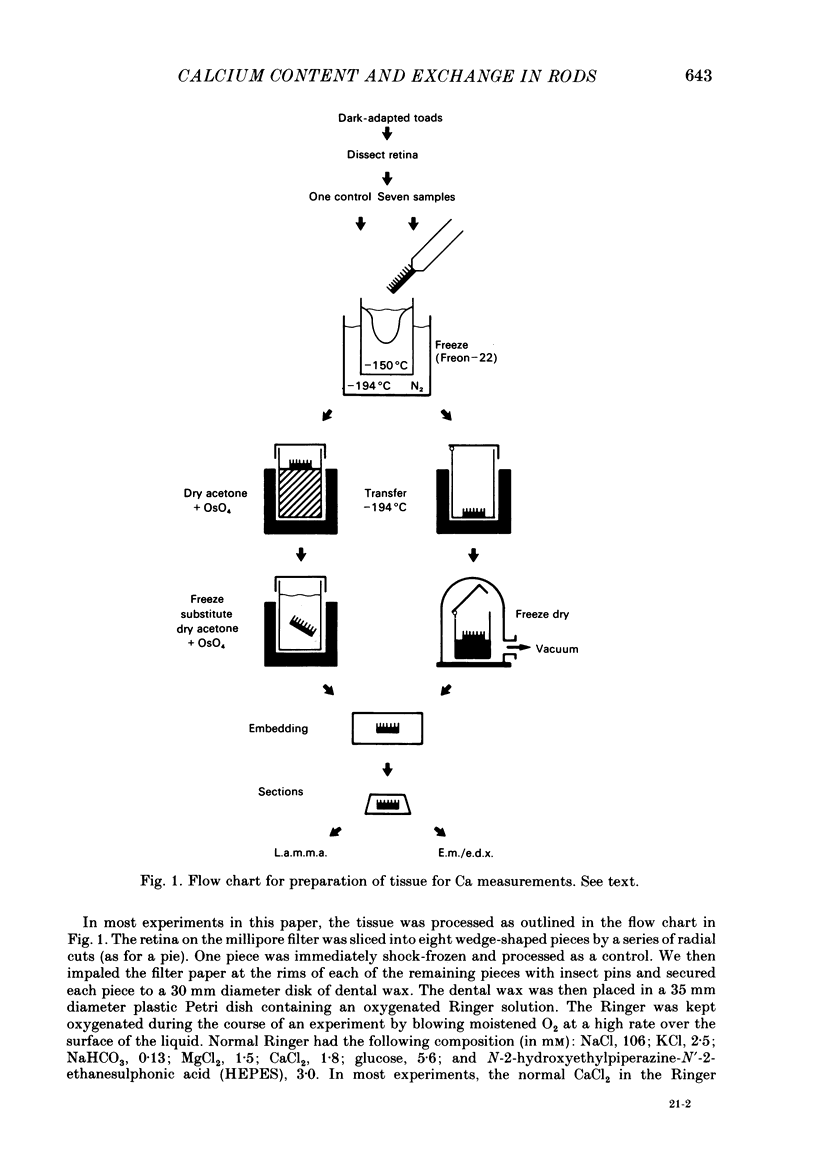
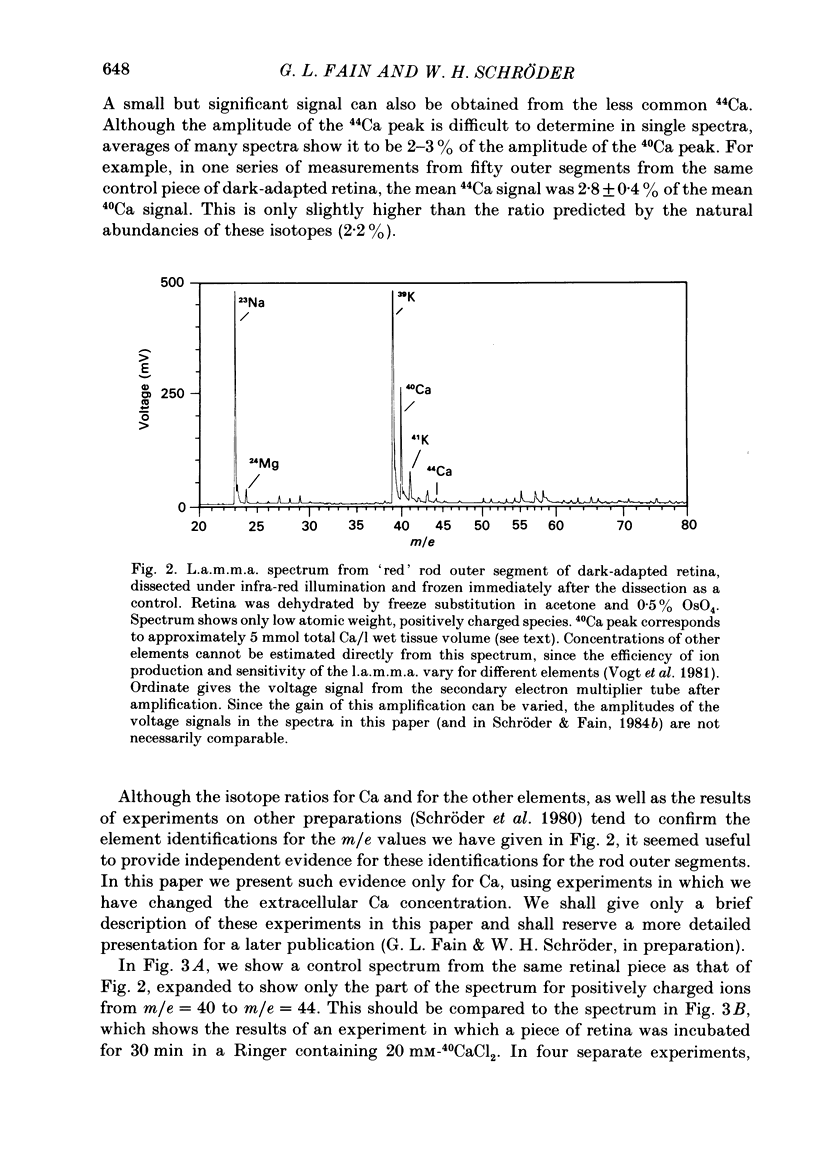
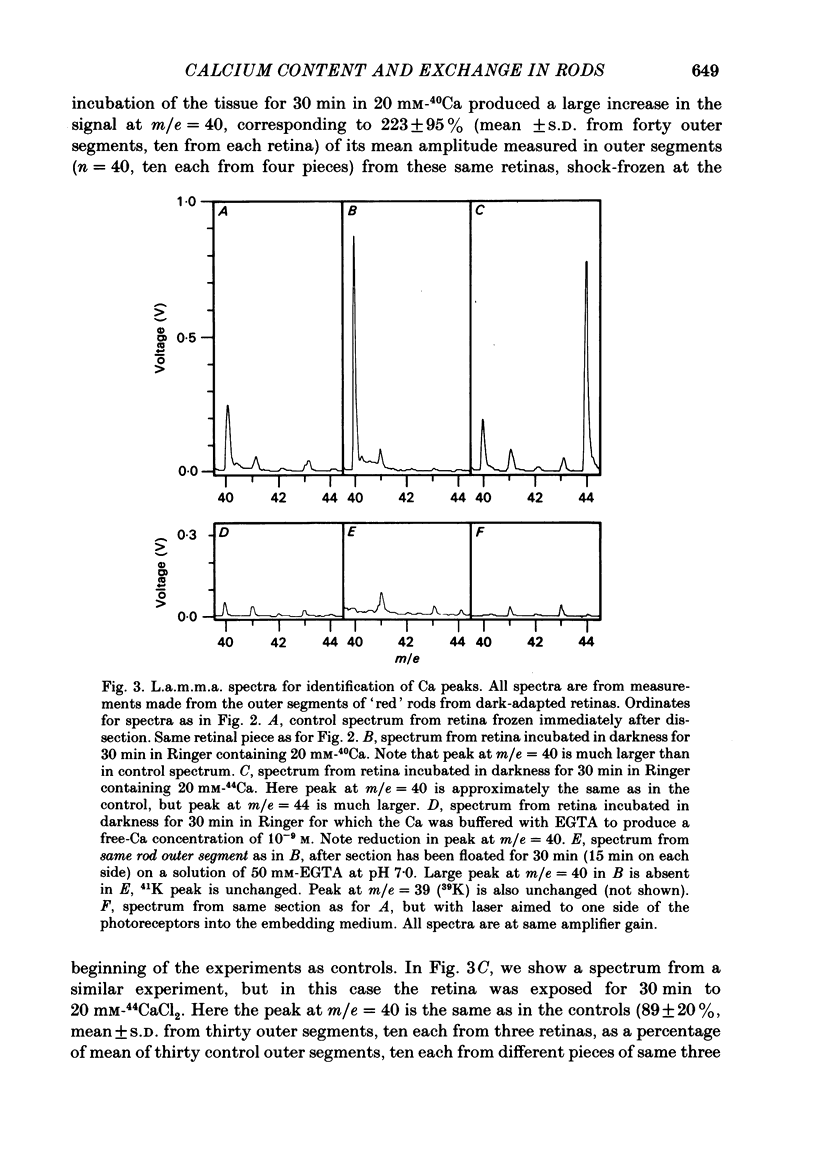
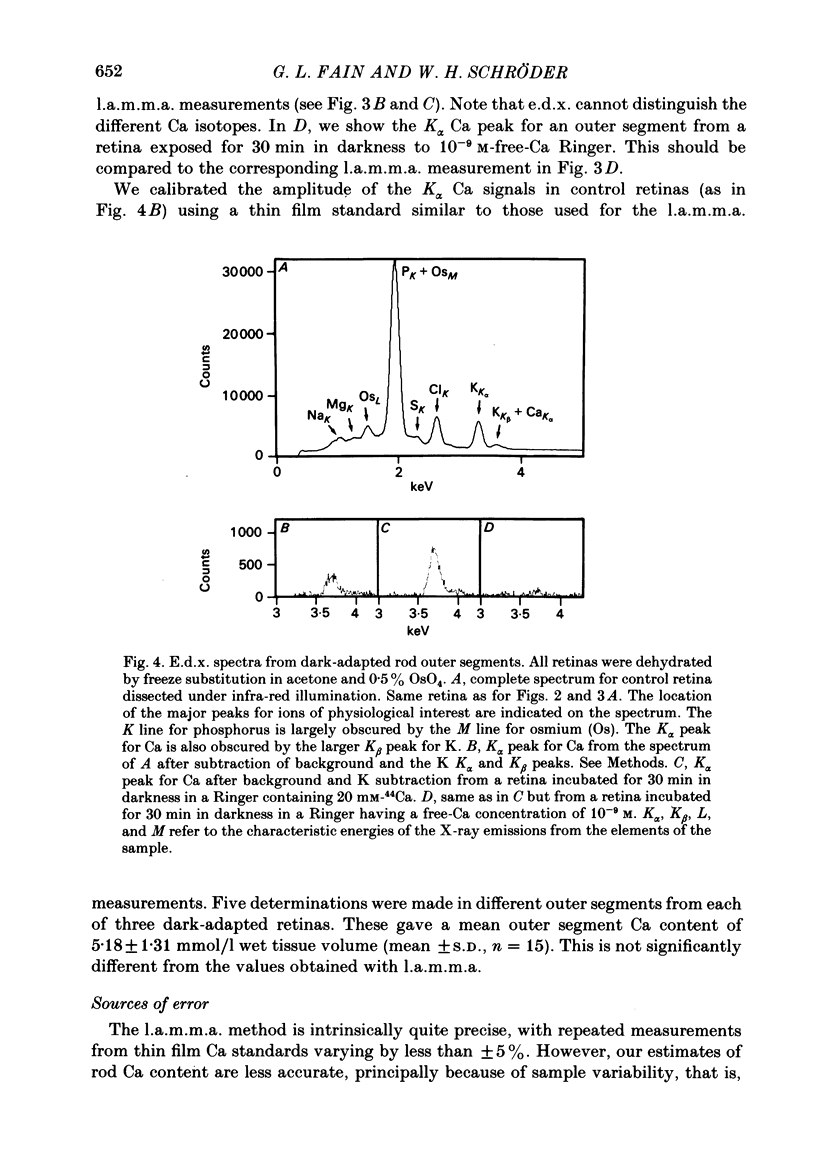
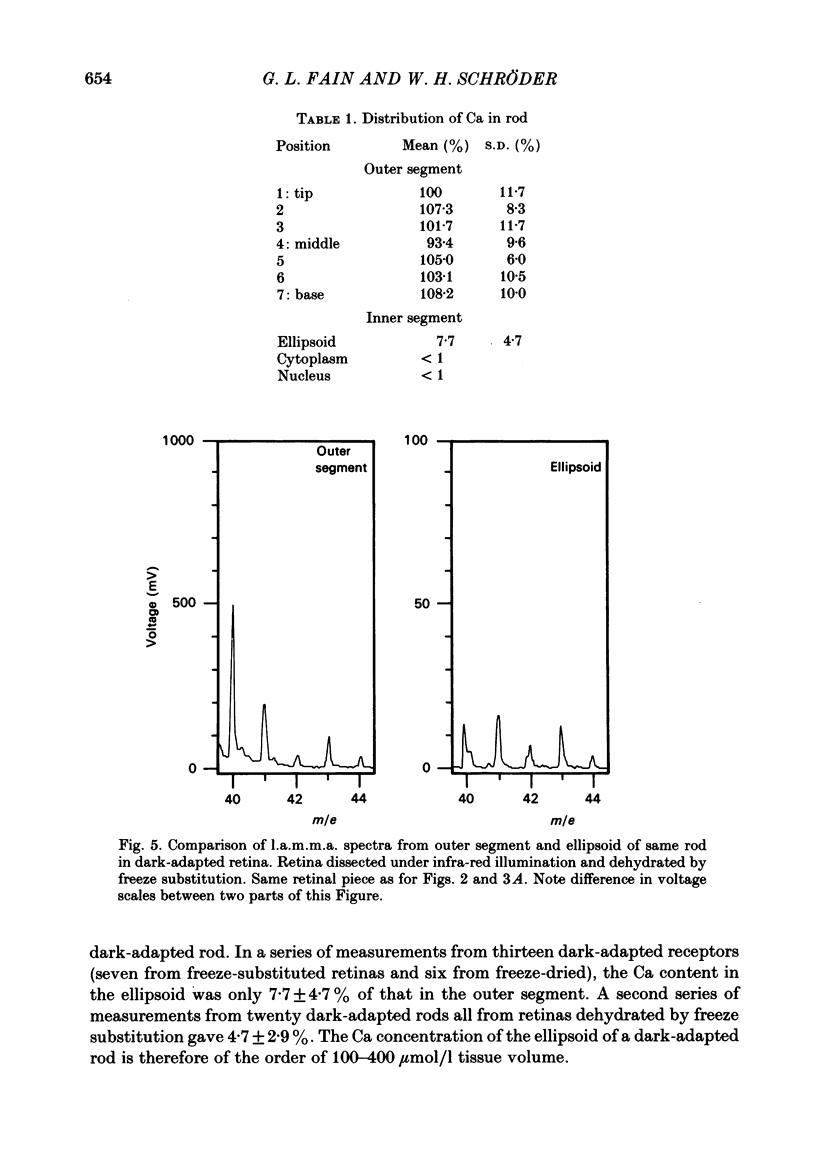
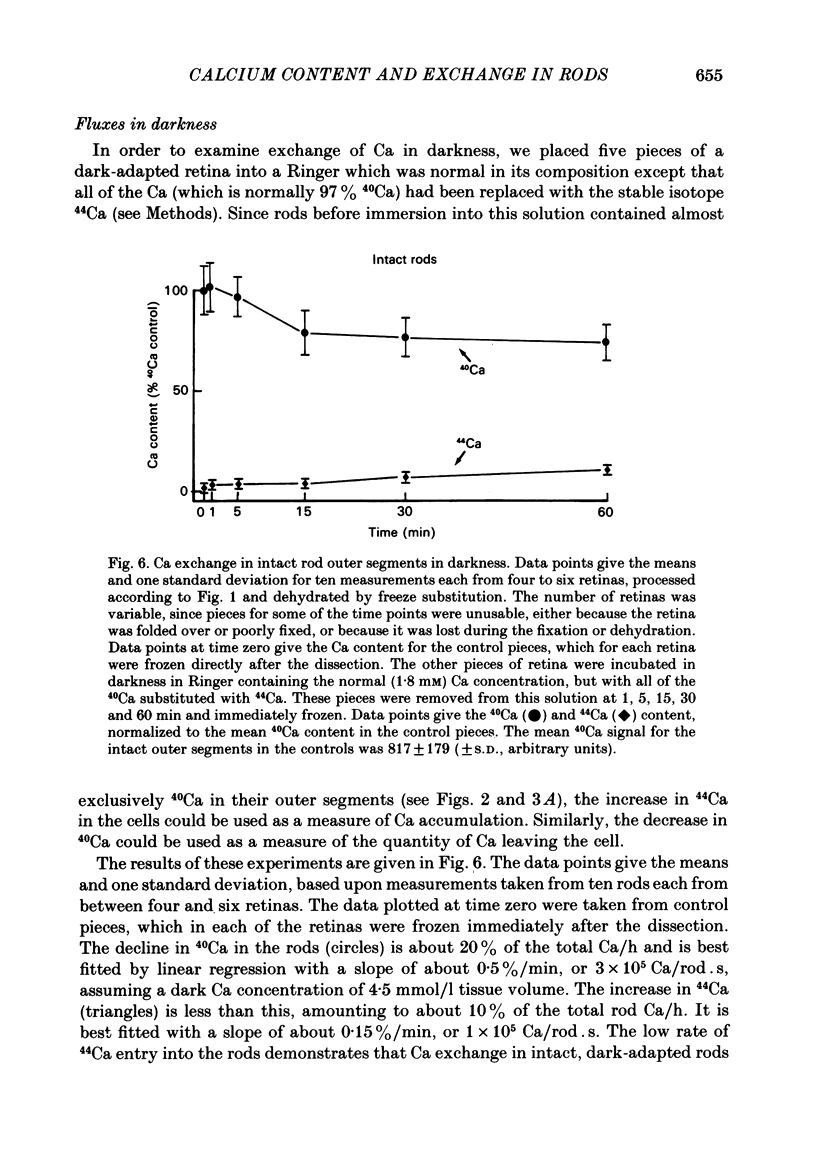
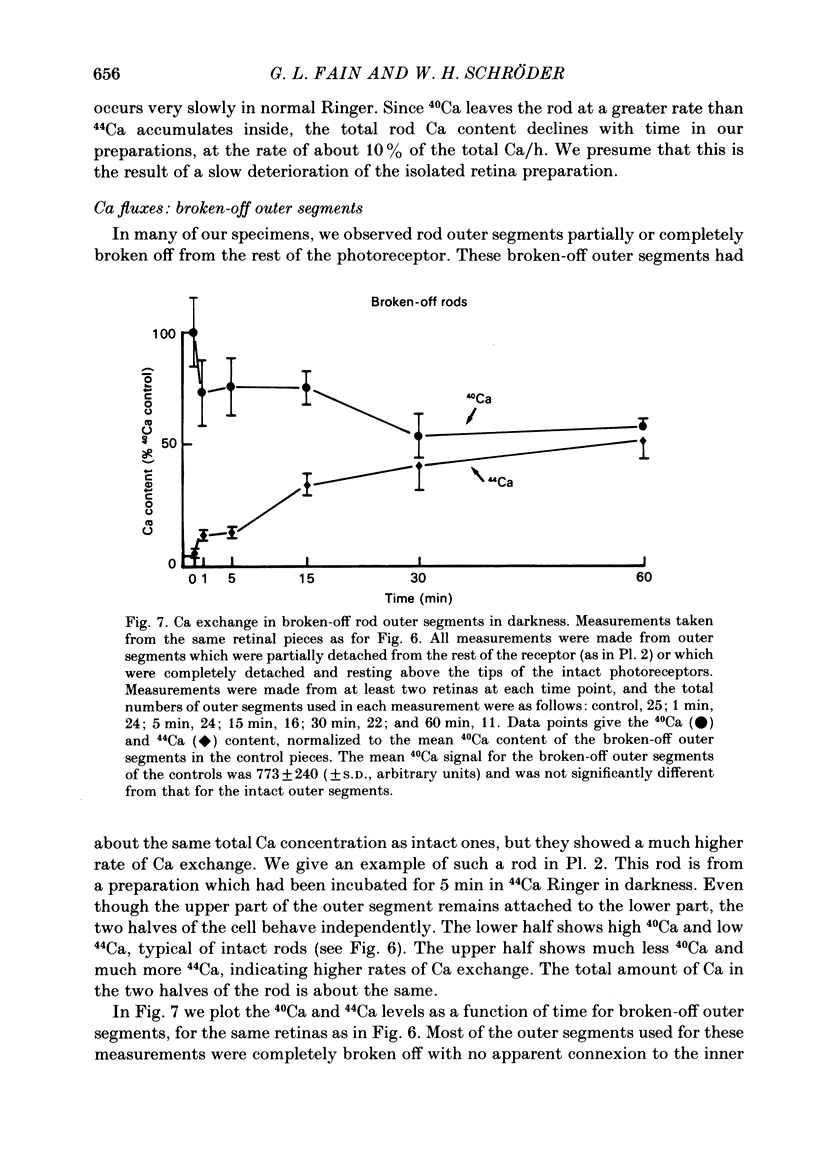
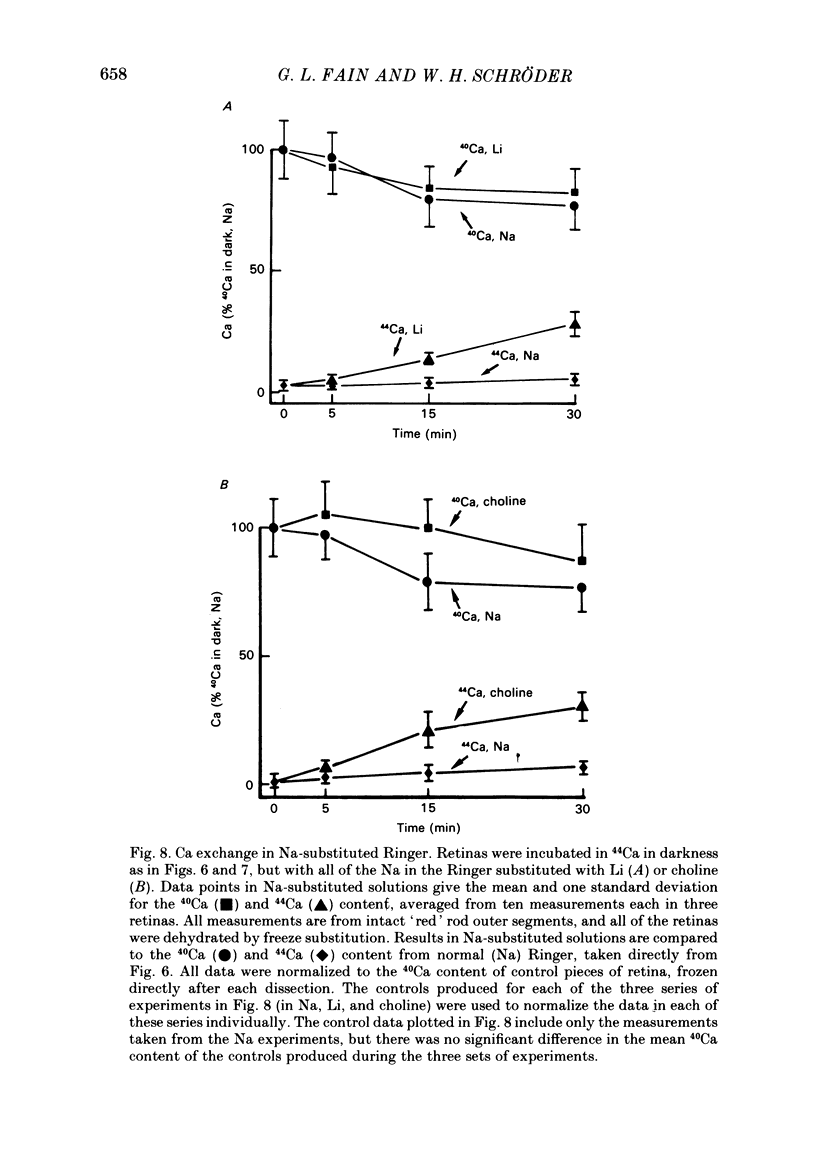
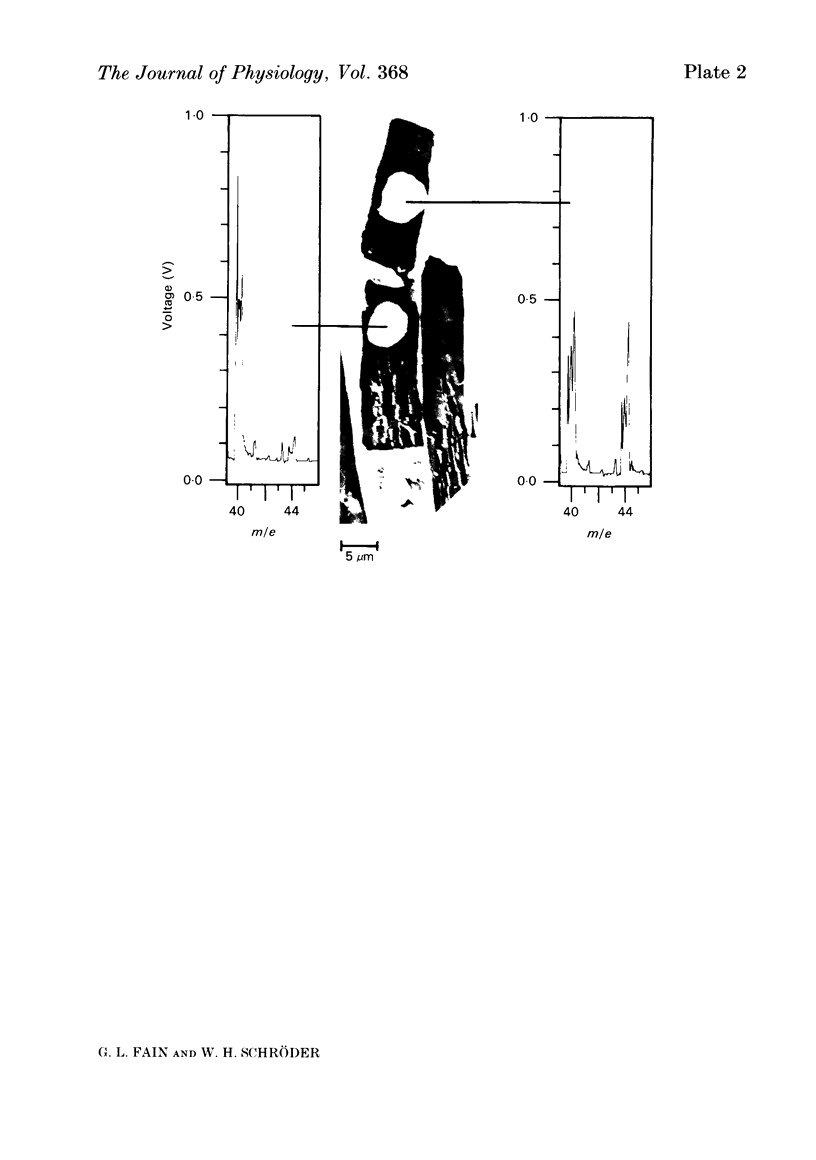
We have used laser-activated micro mass analysis (l.a.m.m.a.) and energy-dispersive X-ray analysis (e.d.x.) to measure Ca content and Ca movements in 'red' rod photoreceptors in the dark-adapted retina of the toad, Bufo marinus. Measurements with both l.a.m.m.a. and e.d.x. show that intact rod outer segments contain 4-5 mmol total Ca/l wet tissue volume, or 1-2 Ca per rhodopsin. We could detect no significant variation in the total Ca as a function of distance across or up and down the outer segment. In the inner segment, Ca could be detected only within the mitochondria-rich ellipsoid body, where the total Ca concentration was of the order of 100-400 mumol/l wet tissue volume. To measure the exchange of Ca in outer segments from intact photoreceptors, we exposed the dark-adapted retina to Ringer containing the stable isotope 44Ca. Since l.a.m.m.a. can measure separately the concentrations of each of the isotopes of the elements, and since native rods contain almost exclusively 40Ca, the increase in 44Ca and decrease in 40Ca could be used as a measure of Ca influx and efflux. Ca exchange in intact rod outer segments in darkness is very slow. The rate of accumulation of 44Ca was only 10(5) Ca/rod.s, or about 10% of the total outer segment Ca/h. This slow rate of exchange is apparently not the result of restricted movement of Ca across the plasma membrane. Ca exchange was also measured in outer segments which were either partially or entirely detached from the rest of the photoreceptor. In broken-off outer segments, Ca exchange is faster than in the intact organelles, and in 1 h, half of the 44Ca exchanges for 40Ca. When the retina was incubated in Ringer for which all of the Na was substituted with Li or choline, there was an increase in the rate of 44Ca accumulation in intact outer segments, probably due to an inhibition of Na-Ca counter transport across the plasma membrane. Our measurements indicate that the great majority of the Ca in the rod appears to be inaccessible to exchange under physiological conditions, probably because it is sequestered within the disks which in intact rods appear to be nearly impermeable to Ca in darkness.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian B. L., Fain G. L. The effects of low calcium and background light on the sensitivity of toad rods. J Physiol. 1982 Sep;330:307–329. doi: 10.1113/jphysiol.1982.sp014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Brown J. E. Excitation in vertebrate retinal rods. Soc Gen Physiol Ser. 1979;33:117–121. [PubMed] [Google Scholar]
- Chabre M. X-ray diffraction studies of retinal rods. I. Structure of the disc membrane, effect of illumination. Biochim Biophys Acta. 1975 Mar 25;382(3):322–335. doi: 10.1016/0005-2736(75)90274-6. [DOI] [PubMed] [Google Scholar]
- Cohen A. I. New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J Cell Biol. 1968 May;37(2):424–444. doi: 10.1083/jcb.37.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENTON E. J., WYLLIE J. H. Study of the photosensitive pigments in the pink and green rods of the frog. J Physiol. 1955 Jan 28;127(1):81–89. doi: 10.1113/jphysiol.1955.sp005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Schröder W. H. Calcium content and light-induced release from photoreceptors: measurements with laser micro-mass analysis. Prog Clin Biol Res. 1985;176:3–20. [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Robinson W. E., Yoshikami S. Ionic aspects of excitation in rod outer segments. Ciba Found Symp. 1975;(31):169–189. doi: 10.1002/9780470720134.ch10. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hess H. H. The high calcium content of retinal pigmented epithelium. Exp Eye Res. 1975 Nov;21(5):471–479. doi: 10.1016/0014-4835(75)90128-1. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P. Calcium metabolism in vertebrate photoreceptors. Cell Calcium. 1982 May;3(2):83–112. doi: 10.1016/0143-4160(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Korenbrot J. I. Signal mechanisms of phototransduction in retinal rod. CRC Crit Rev Biochem. 1985;17(3):223–256. doi: 10.3109/10409238509113605. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties A. M., Liebman P. A. Cones of living amphibian eye: selective staining. Science. 1970 Jun 19;168(3938):1475–1477. doi: 10.1126/science.168.3938.1475. [DOI] [PubMed] [Google Scholar]
- Liebman P. A. Rod disk calcium movement and transduction: a poorly illuminated story. Ann N Y Acad Sci. 1978 Apr 28;307:642–644. doi: 10.1111/j.1749-6632.1978.tb41987.x. [DOI] [PubMed] [Google Scholar]
- Nöll G., Stieve H., Winterhager J. Interaction of bovine rhodopsin with calcium ions. II: calcium release in bovine rod outer segments upon bleaching. Biophys Struct Mech. 1979 Mar 21;5(1):43–53. doi: 10.1007/BF00535772. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Reese T. S. A freeze-substitution method for localizing divalent cations: examples from secretory systems. Fed Proc. 1980 Aug;39(10):2802–2808. [PubMed] [Google Scholar]
- Requena J. Calcium transport and regulation in nerve fibers. Annu Rev Biophys Bioeng. 1983;12:237–257. doi: 10.1146/annurev.bb.12.060183.001321. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Calcium translocation and storage of isolated intact cattle rod outer segments in darkness. Biochim Biophys Acta. 1979 Jul 5;554(2):441–459. doi: 10.1016/0005-2736(79)90383-3. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Ion selectivity of the cation transport system of isolated intact cattle rod outer segments: evidence for a direct communication between the rod plasma membrane and the rod disk membranes. Biochim Biophys Acta. 1980 May 8;598(1):66–90. doi: 10.1016/0005-2736(80)90266-7. [DOI] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Schröder W., Frings D., Stieve H. Measuring calcium uptake and release by invertebrate photoreceptor cells by laser microprobe mass spectroscopy. Scan Electron Microsc. 1980;(Pt 2):647-54, 606. [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Walz B. Elemental distribution in Rana pipiens retinal rods: quantitative electron probe analysis. J Physiol. 1985 Jan;358:183–195. doi: 10.1113/jphysiol.1985.sp015547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuts E. Z. Calcium flux across disk membranes. Studies with intact rod photoreceptors and purified disks. J Gen Physiol. 1980 Sep;76(3):253–286. doi: 10.1085/jgp.76.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuts E. Z., Cone R. A. Calcium content of frog rod outer segments and discs. Biochim Biophys Acta. 1977 Jul 14;468(2):194–208. doi: 10.1016/0005-2736(77)90114-6. [DOI] [PubMed] [Google Scholar]
- Ungar F., Piscopo I., Letizia J., Holtzman E. Uptake of calcium by the endoplasmic reticulum of the frog photoreceptor. J Cell Biol. 1984 May;98(5):1645–1655. doi: 10.1083/jcb.98.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]




