Abstract
The flux of thiamine from the blood into the brain has been measured by a specially devised procedure in which a steady raised level of the vitamin, with or without radioactive labelling, was achieved rapidly and maintained steadily in the circulating blood plasma. This was done by a single rapid I.V. injection followed by a continuous injection given at a rate adjusted according to a pre-determined programme, so as to replace the injected material at the rate at which it had been found to leave the circulation in preliminary experiments. A series of four chemical analogues of thiamine were given to see how each affected the flux of thiamine into the brain and the findings are compared with those for a fifth analogue studied in previous work. Pyrithiamine, thiamine disulphide and acetylthiamine, like amprolium, inhibited thiamine transport across the blood-brain barrier. Kinetic analysis shows that they compete mainly for the saturable component of thiamine flux across the blood-brain barrier, with only a slight inhibition of the non-saturable component, most clearly seen with pyrithiamine. Oxythiamine, despite its close chemical similarity to thiamine did not have any significant effect upon the flux of the vitamin into the brain. These findings help to explain the efficacy of pyrithiamine administration, especially in conjunction with a thiamine-deficient diet, in rapidly producing central neurological signs of deficiency.
Full text
PDF


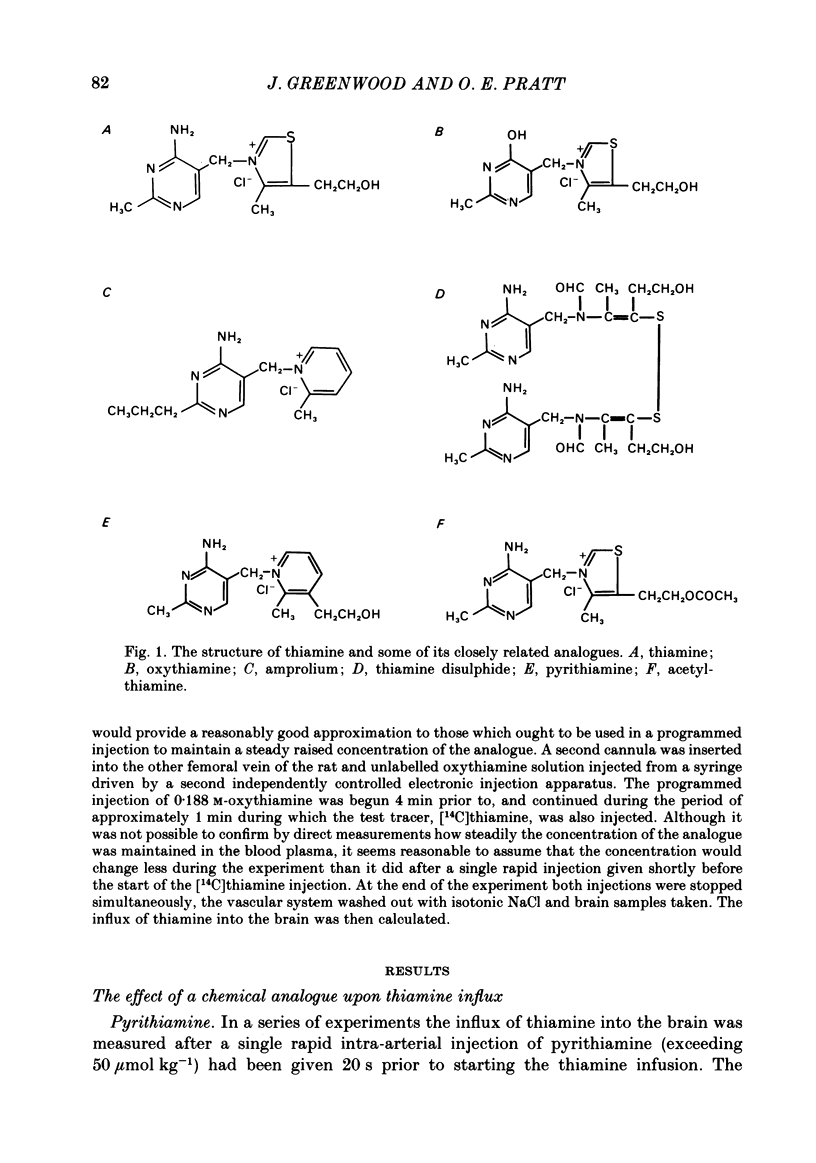

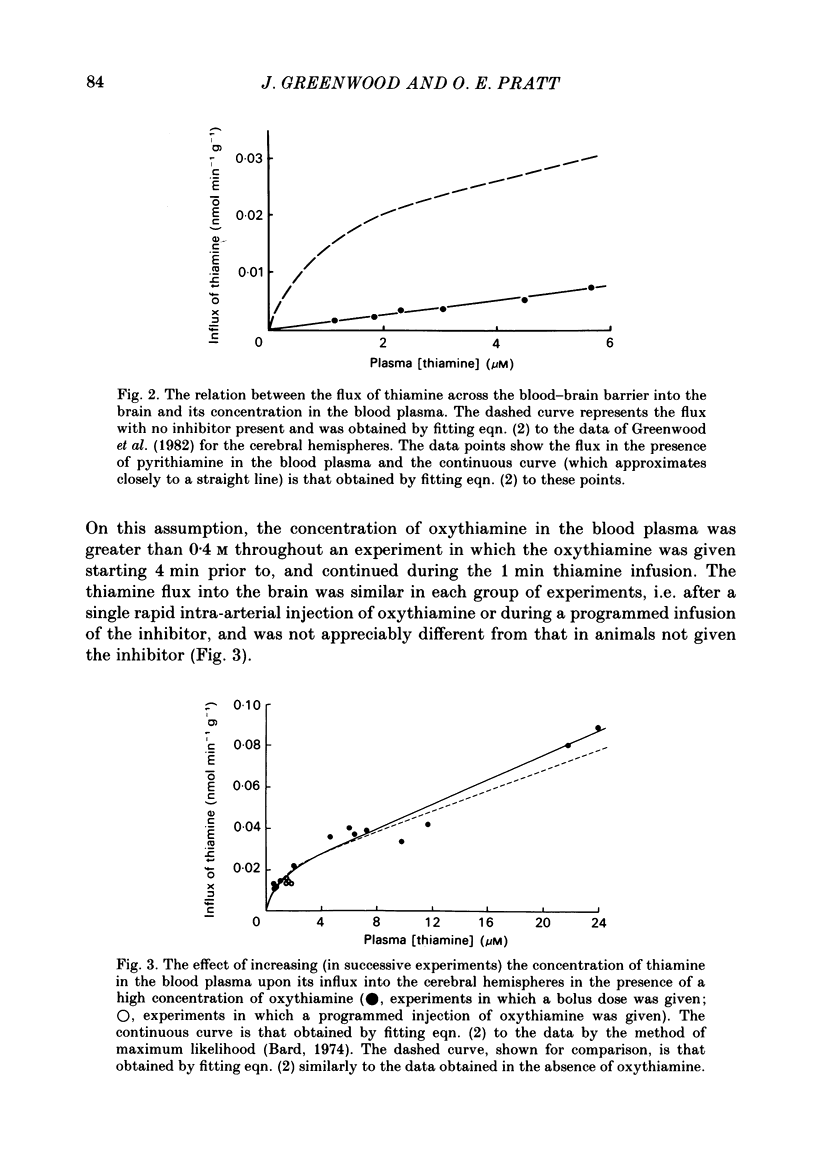
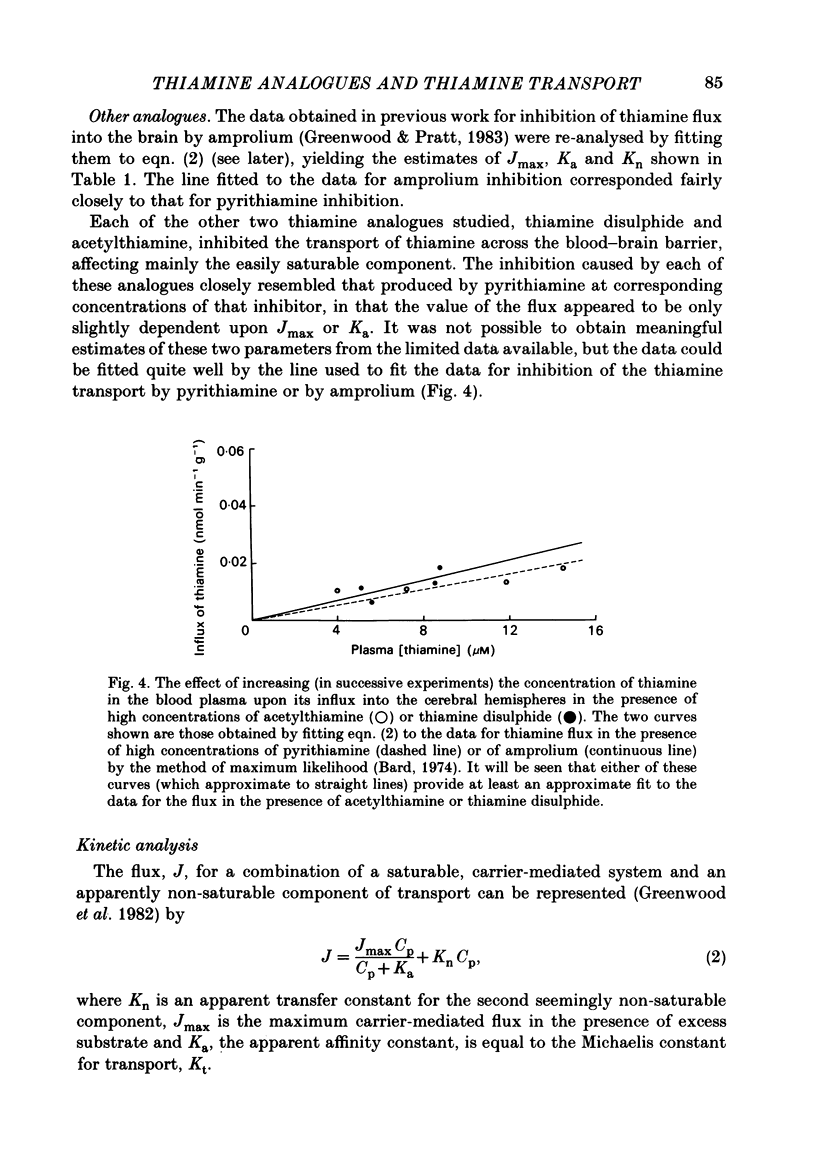






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa H., Watanabe I. S., Furuse T., Iwasaki Y., Satoyoshi E., Sumi T., Moroji T. Low energy levels in thiamine-deficient encephalopathy. J Neuropathol Exp Neurol. 1984 May;43(3):276–287. doi: 10.1097/00005072-198405000-00006. [DOI] [PubMed] [Google Scholar]
- BRIN M. Effects of thiamine deficiency and of oxythiamine on rat tissue transketolase. J Nutr. 1962 Oct;78:179–183. doi: 10.1093/jn/78.2.179. [DOI] [PubMed] [Google Scholar]
- Collins R. C., Kirkpatrick J. B., McDougal D. B., Jr Some regional pathologic and metabolic consequences in mouse brain of pyrithiamine-induced thiamine deficiency. J Neuropathol Exp Neurol. 1970 Jan;29(1):57–69. doi: 10.1097/00005072-197001000-00005. [DOI] [PubMed] [Google Scholar]
- DE CARO L., RINDI G., PERRI V., FERRARI G. Avitaminosi B1 alimentare e da antivitamine (neopiritiamina ed ossitiamina) nei roditori. Int Z Vitaminforsch Beih. 1958;28(3):252–273. [PubMed] [Google Scholar]
- Daniel P. M., Donaldson J., Pratt O. E. A method for injecting substances into the circulation to reach rapidly and to maintain a steady level. With examples of its application in the study of carbohydrate and amino acid metabolism. Med Biol Eng. 1975 Mar;13(2):214–227. doi: 10.1007/BF02477731. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Love E. R., Moorhouse S. R., Pratt O. E., Wilson P. A method for rapidly washing the blood out of an organ or tissue of the anaesthetized living animal. J Physiol. 1974 Mar;237(2):11P–12P. [PubMed] [Google Scholar]
- Deane B. R., Greenwood J., Lantos P. L., Pratt O. E. The vasculature of experimental brain tumours. Part 4. The quantification of vascular permeability. J Neurol Sci. 1984 Jul;65(1):59–68. doi: 10.1016/0022-510x(84)90067-4. [DOI] [PubMed] [Google Scholar]
- Greenwood J., Love E. R., Pratt O. E. Kinetics of thiamine transport across the blood-brain barrier in the rat. J Physiol. 1982 Jun;327:95–103. doi: 10.1113/jphysiol.1982.sp014222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J., Pratt O. E. Inhibition of thiamine transport across the blood-brain barrier in the rat by a chemical analogue of the vitamin. J Physiol. 1983 Mar;336:479–486. doi: 10.1113/jphysiol.1983.sp014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim A. M., Pappius H. M. Sequence of metabolic, clinical, and histological events in experimental thiamine deficiency. Ann Neurol. 1983 Apr;13(4):365–375. doi: 10.1002/ana.410130403. [DOI] [PubMed] [Google Scholar]
- Hoyumpa A. M., Jr, Middleton H. M., 3rd, Wilson F. A., Schenker S. Thiamine transport across the rat intestine. I. Normal characteristics. Gastroenterology. 1975 May;68(5 Pt 1):1218–1227. [PubMed] [Google Scholar]
- Hoyumpa A. M., Jr, Strickland R., Sheehan J. J., Yarborough G., Nichols S. Dual system of intestinal thiamine transport in humans. J Lab Clin Med. 1982 May;99(5):701–708. [PubMed] [Google Scholar]
- Irle E., Markowitsch H. J. Thiamine deficiency in the cat leads to severe learning deficits and to widespread neuroanatomical damage. Exp Brain Res. 1982;48(2):199–208. doi: 10.1007/BF00237215. [DOI] [PubMed] [Google Scholar]
- Markson L. M., Lewis G., Terlecki S., Edwin E. E., Ford J. E. The aetiology of cerebrocortical necrosis: the effects of administering antimetabolites of thiamine to preruminant calves. Br Vet J. 1972 Oct;128(10):488–499. doi: 10.1016/s0007-1935(17)36733-7. [DOI] [PubMed] [Google Scholar]
- Menon I. A., Quastel J. H. Transport and metabolism of thiamine in Ehrlich ascites-carcinoma cells. Biochem J. 1966 Jun;99(3):766–775. doi: 10.1042/bj0990766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock D. S., Gubler C. J. Effects of thiamine deficiency and treatment with the antagonists, oxythiamine and pyrithiamine, on the levels and distribution of thiamine derivatives in rat brain. J Nutr Sci Vitaminol (Tokyo) 1973;19(3):237–249. doi: 10.3177/jnsv.19.237. [DOI] [PubMed] [Google Scholar]
- Pratt O. E. An electronically controlled syringe drive for giving an injection at a variable rate according to a preset programme. J Physiol. 1974 Mar;237(2):5P–6P. [PubMed] [Google Scholar]
- RINDI G., DEGIUSEPPE L., VENTURA U. DISTRIBUTION AND PHOSPHORYLATION OF OXYTHIAMINE IN RAT TISSUES. J Nutr. 1963 Oct;81:147–154. doi: 10.1093/jn/81.2.147. [DOI] [PubMed] [Google Scholar]
- RINDI G., PERRI V. Uptake of pyrithiamine by tissue of rats. Biochem J. 1961 Jul;80:214–216. doi: 10.1042/bj0800214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINDI G., PERRIV, DE CAROL The uptake of pyrithiamine by cerebral tissue. Experientia. 1961 Dec 15;17:546–547. doi: 10.1007/BF02156413. [DOI] [PubMed] [Google Scholar]
- Reggiani C., Patrini C., Rindi G. Nervous tissue thiamine metabolism in vivo. I. Transport of thiamine and thiamine monophosphate from plasma to different brain regions of the rat. Brain Res. 1984 Feb 20;293(2):319–327. doi: 10.1016/0006-8993(84)91239-3. [DOI] [PubMed] [Google Scholar]
- Rindi G., De Giuseppe L., Sciorelli G. Thiamine monophosphate, a normal constituent of rat plasma. J Nutr. 1968 Apr;94(4):447–454. doi: 10.1093/jn/94.4.447. [DOI] [PubMed] [Google Scholar]
- Rindi G., Ventura U. Phosphorylation and uphill intestinal transport of thiamine, in vitro. Experientia. 1967 Mar 15;23(3):175–176. doi: 10.1007/BF02136267. [DOI] [PubMed] [Google Scholar]
- Rogers E. F. General discussion of antithiamin compounds and thiamin antagonists. Ann N Y Acad Sci. 1982;378:157–160. doi: 10.1111/j.1749-6632.1982.tb31194.x. [DOI] [PubMed] [Google Scholar]
- SHARMA S. K., QUASTEL J. H. TRANSPORT AND METABOLISM OF THIAMINE IN RAT BRAIN CORTEX IN VITRO. Biochem J. 1965 Mar;94:790–800. doi: 10.1042/bj0940790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R. Thiamine transport in the central nervous system. Am J Physiol. 1976 Apr;230(4):1101–1107. doi: 10.1152/ajplegacy.1976.230.4.1101. [DOI] [PubMed] [Google Scholar]
- Troncoso J. C., Johnston M. V., Hess K. M., Griffin J. W., Price D. L. Model of Wernicke's encephalopathy. Arch Neurol. 1981 Jun;38(6):350–354. doi: 10.1001/archneur.1981.00510060052007. [DOI] [PubMed] [Google Scholar]
- Watanabe I. Pyrithiamine-induced acute thiamine-deficient encephalopathy in the mouse. Exp Mol Pathol. 1978 Jun;28(3):381–394. doi: 10.1016/0014-4800(78)90012-6. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Tomita T., Hung K. S., Iwasaki Y. Edematous necrosis in thiamine-deficient encephalopathy of the mouse. J Neuropathol Exp Neurol. 1981 Jul;40(4):454–471. doi: 10.1097/00005072-198107000-00008. [DOI] [PubMed] [Google Scholar]


