Abstract
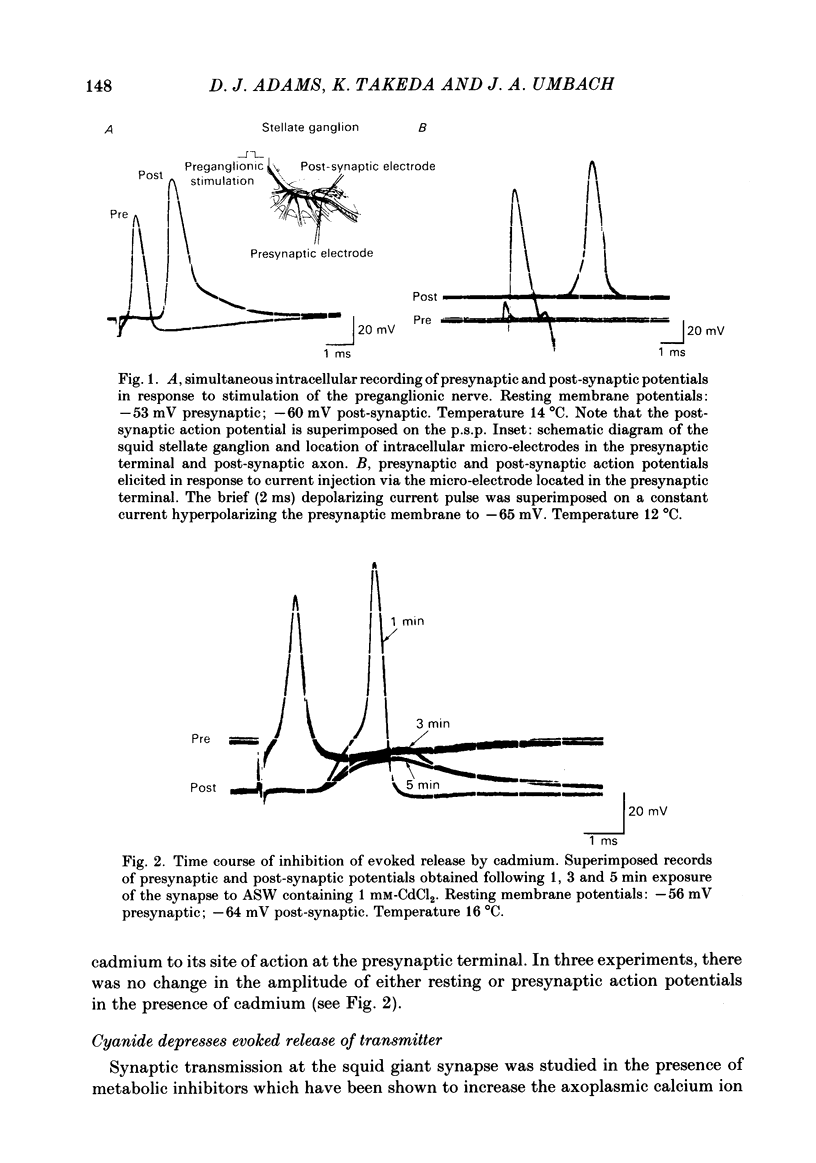
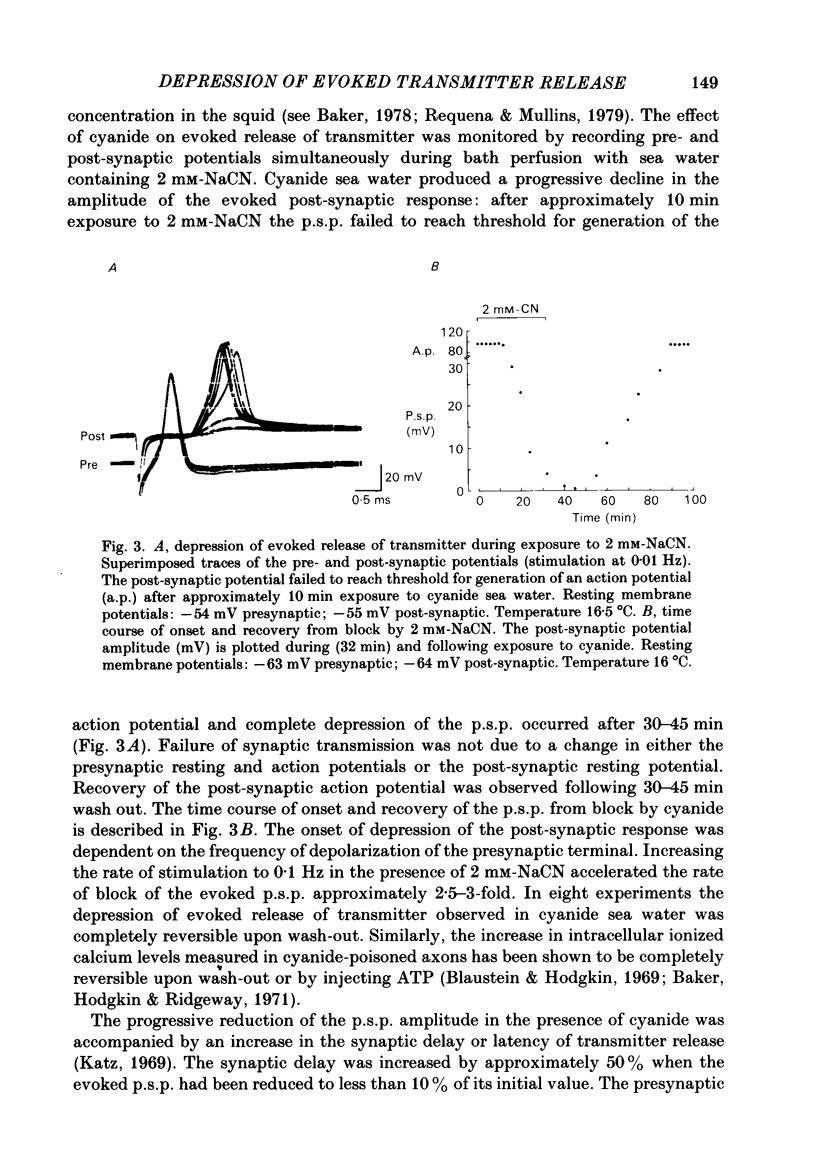
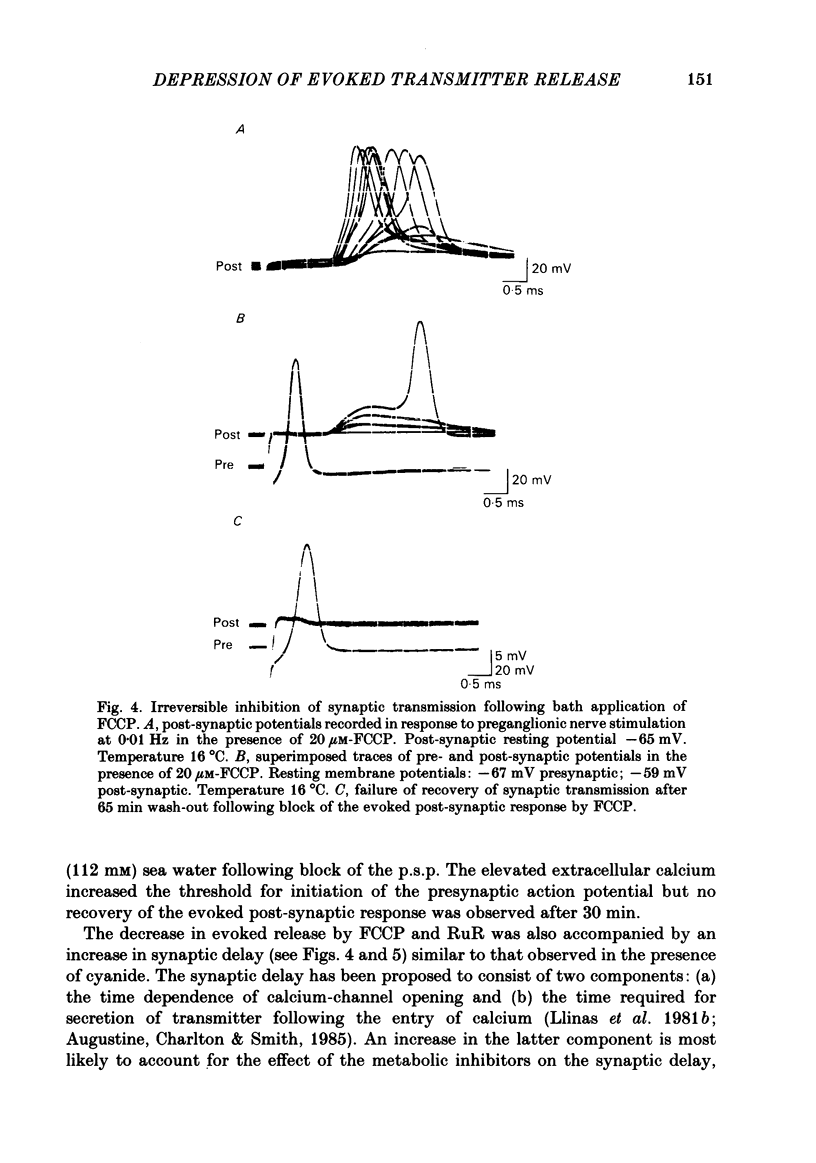
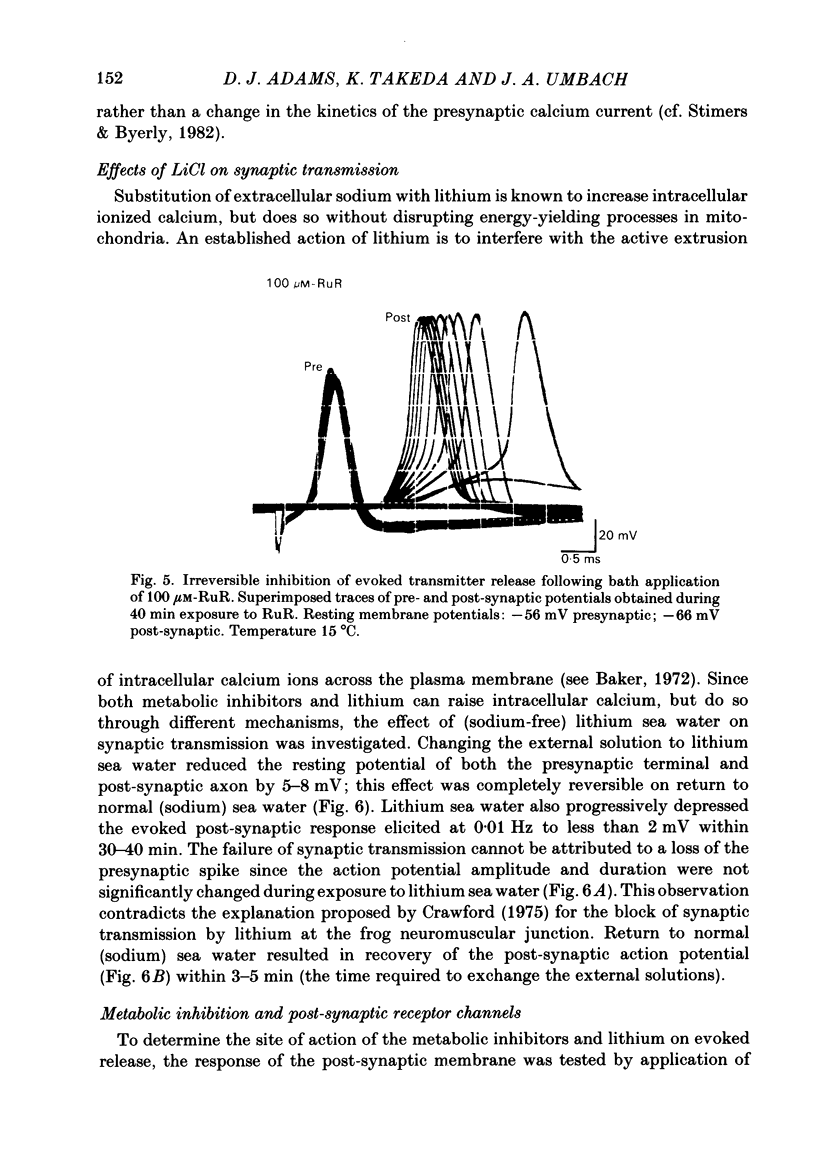
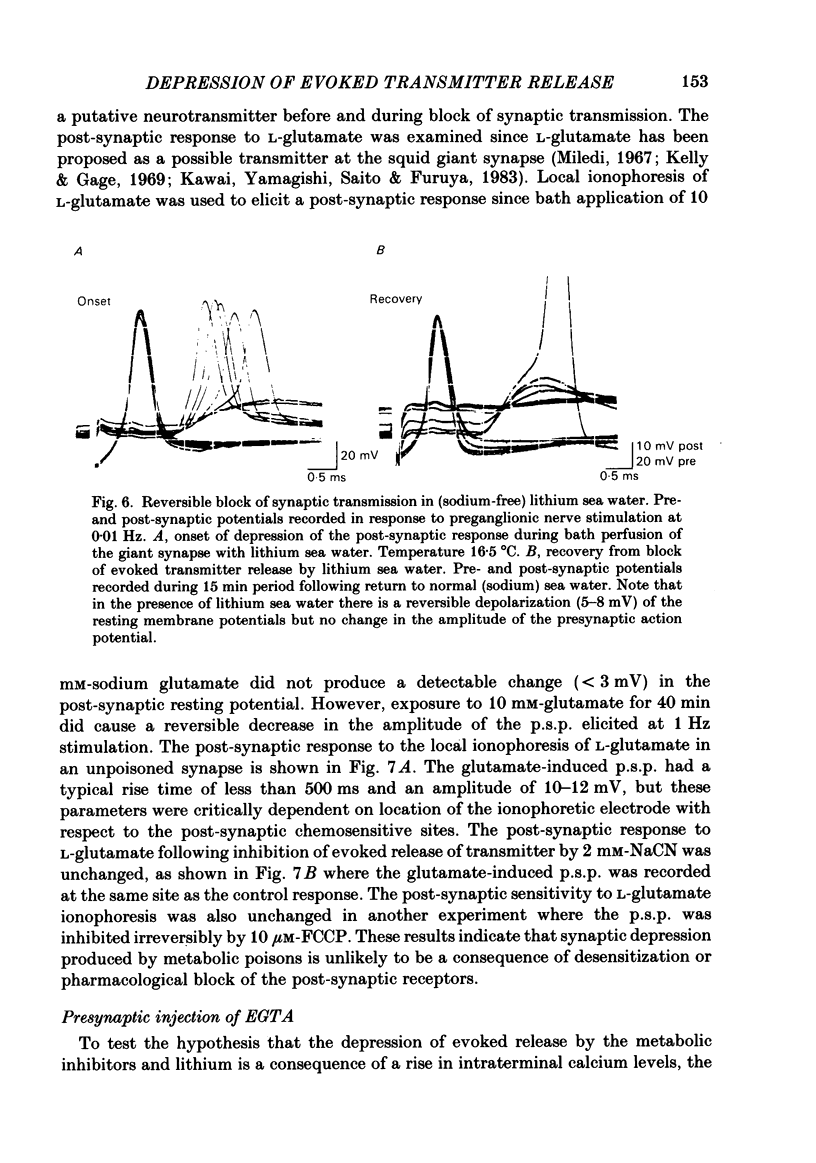
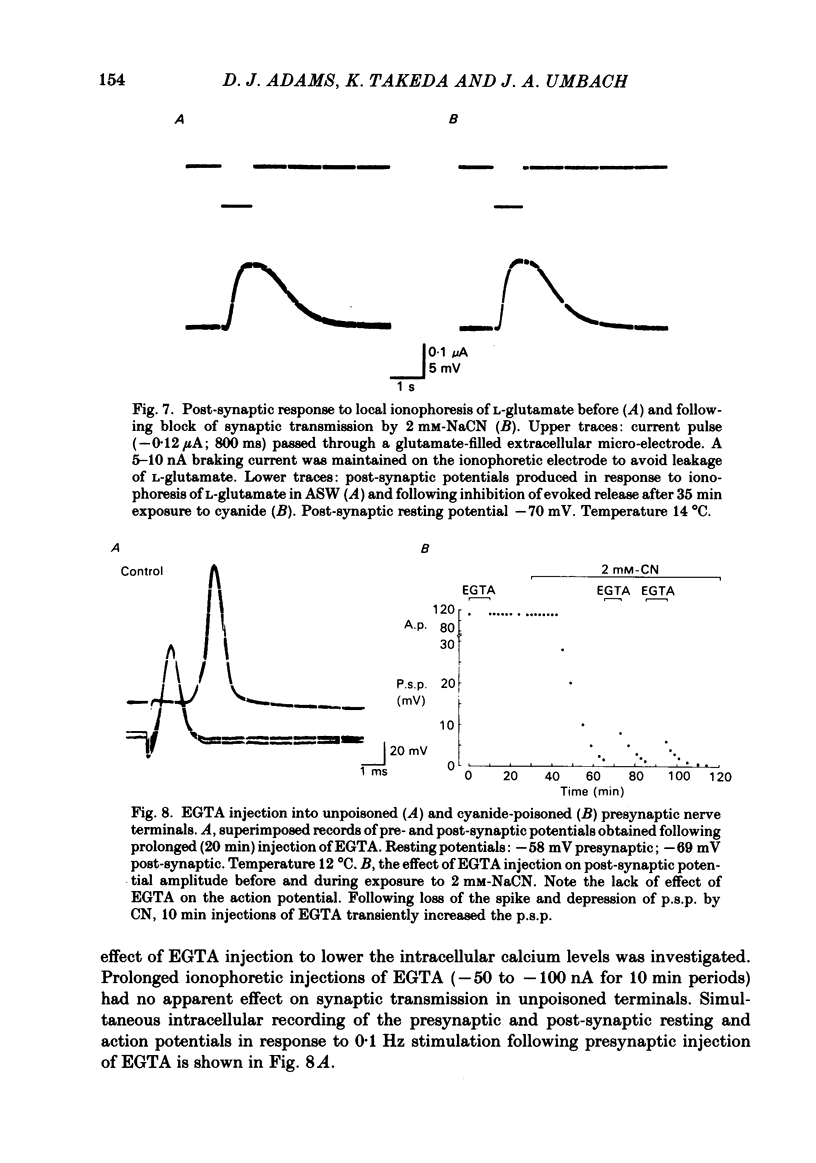
Evoked release of transmitter at the squid giant synapse was examined under conditions where the calcium ion concentration in the presynaptic terminal was manipulated by inhibitors of calcium sequestration. Simultaneous intracellular recordings of presynaptic and post-synaptic resting and action potentials were made during bath application of one of the following metabolic inhibitors: sodium cyanide (NaCN), carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone (FCCP); ruthenium red (RuR) and sodium-free (lithium) sea water. Cyanide and lithium sea water reversibly depressed the post-synaptic potential (p.s.p.) whilst RuR and FCCP blocked the evoked post-synaptic response irreversibly. The progressive reduction of p.s.p. amplitude was accompanied by a reversible increase in synaptic delay. The time course of block of the p.s.p. was similar for different agents and dependent on the rate of presynaptic activity (30-40 min at 0.01 Hz). Recovery of the post-synaptic action potential following block by cyanide and lithium sea water was obtained within 40 min and 5 min respectively. Synaptic depression by the metabolic inhibitors does not result from changes in presynaptic resting or action potentials, nor from a change in post-synaptic receptor sensitivity. The post-synaptic response to the local ionophoresis of L-glutamate was unchanged following inhibition of evoked release of transmitter by cyanide. Injections of EGTA into presynaptic terminals poisoned by cyanide produced transient increases in p.s.p. amplitude, suggesting that cyanide is having its effect through raising intracellular calcium rather than lowering ATP. Control experiments injecting EGTA into unpoisoned nerve terminals showed no apparent effect on evoked transmitter release.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Eckert R. Divalent cations differentially support transmitter release at the squid giant synapse. J Physiol. 1984 Jan;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT S. H. Transmission in squid giant synapses: the importance of oxygen supply and the effects of drugs. J Gen Physiol. 1958 Jan 20;41(3):473–484. doi: 10.1085/jgp.41.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. W. Uptake and binding of calcium by axoplasm isolated from giant axons of Loligo and Myxicola. J Physiol. 1978 Mar;276:103–125. doi: 10.1113/jphysiol.1978.sp012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. The regulation of intracellular calcium in giant axons of Loligo and Myxicola. Ann N Y Acad Sci. 1978 Apr 28;307:250–268. doi: 10.1111/j.1749-6632.1978.tb41956.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Baux G., Simonneau M., Tauc L. Transmitter release: ruthenium red used to demonstrate a possible role of sialic acid containing substrates. J Physiol. 1979 Jun;291:161–178. doi: 10.1113/jphysiol.1979.sp012805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Kendrick N. K. The regulation of intracellular calcium in presynaptic nerve terminals. Ann N Y Acad Sci. 1978 Apr 28;307:195–212. doi: 10.1111/j.1749-6632.1978.tb41943.x. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Active proton transport stimulated by CO2/HCO3-, blocked by cyanide. Nature. 1976 Jan 22;259(5540):240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. Lithium ions and the release of transmitter at the frog neuromuscular junction. J Physiol. 1975 Mar;246(1):109–142. doi: 10.1113/jphysiol.1975.sp010882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Rojas H., Vergara J., Lopez R., Caputo C. Measurements of intracellular ionized calcium in squid giant axons using calcium-selective electrodes. Biochim Biophys Acta. 1983 Mar 9;728(3):311–318. doi: 10.1016/0005-2736(83)90500-x. [DOI] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I. The effect of repetitive stimulation on the passive electrical properties of the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):293–306. doi: 10.1098/rspb.1979.0106. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N., Yamagishi S., Saito M., Furuya K. Blockade of synaptic transmission in the squid giant synapse by a spider toxin (JSTX). Brain Res. 1983 Nov 14;278(1-2):346–349. doi: 10.1016/0006-8993(83)90269-x. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Gage P. W. L-glutamate blockade of transmission at the giant synapse of the squid stellate ganglion. J Neurobiol. 1969;1(2):209–219. doi: 10.1002/neu.480010208. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Mellow A. M., Silinsky E. M. Intracellular magnesium does not antagonize calcium-dependent acetylcholine secretion. J Physiol. 1981 May;314:255–263. doi: 10.1113/jphysiol.1981.sp013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Kusano K. Influence of ionic environment on the relationship between pre- and postsynaptic potentials. J Neurobiol. 1970;1(4):435–437. doi: 10.1002/neu.480010407. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Miledi R. A structural study of the squid synapse after intraaxonal injection of calcium. Proc R Soc Lond B Biol Sci. 1978 Jun 5;201(1145):317–333. doi: 10.1098/rspb.1978.0049. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Calcium transients recorded with arsenazo III in the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):197–211. doi: 10.1098/rspb.1981.0034. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Spontaneous synaptic potentials and quantal release of transmitter in the stellate ganglion of the squid. J Physiol. 1967 Sep;192(2):379–406. doi: 10.1113/jphysiol.1967.sp008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Moody W., Jr Effects of intracellular H+ on the electrical properties of excitable cells. Annu Rev Neurosci. 1984;7:257–278. doi: 10.1146/annurev.ne.07.030184.001353. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., Mullins L. J. Calcium movement in nerve fibres. Q Rev Biophys. 1979 Aug;12(3):371–460. doi: 10.1017/s0033583500005473. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Byerly L. Slowing of sodium current inactivation by ruthenium red in snail neurons. J Gen Physiol. 1982 Oct;80(4):485–497. doi: 10.1085/jgp.80.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach J. A. Changes in intracellular pH affect calcium currents in Paramecium caudatum. Proc R Soc Lond B Biol Sci. 1982 Sep 22;216(1203):209–224. doi: 10.1098/rspb.1982.0071. [DOI] [PubMed] [Google Scholar]
- Walton K., Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983 Nov 14;278(1-2):387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]


