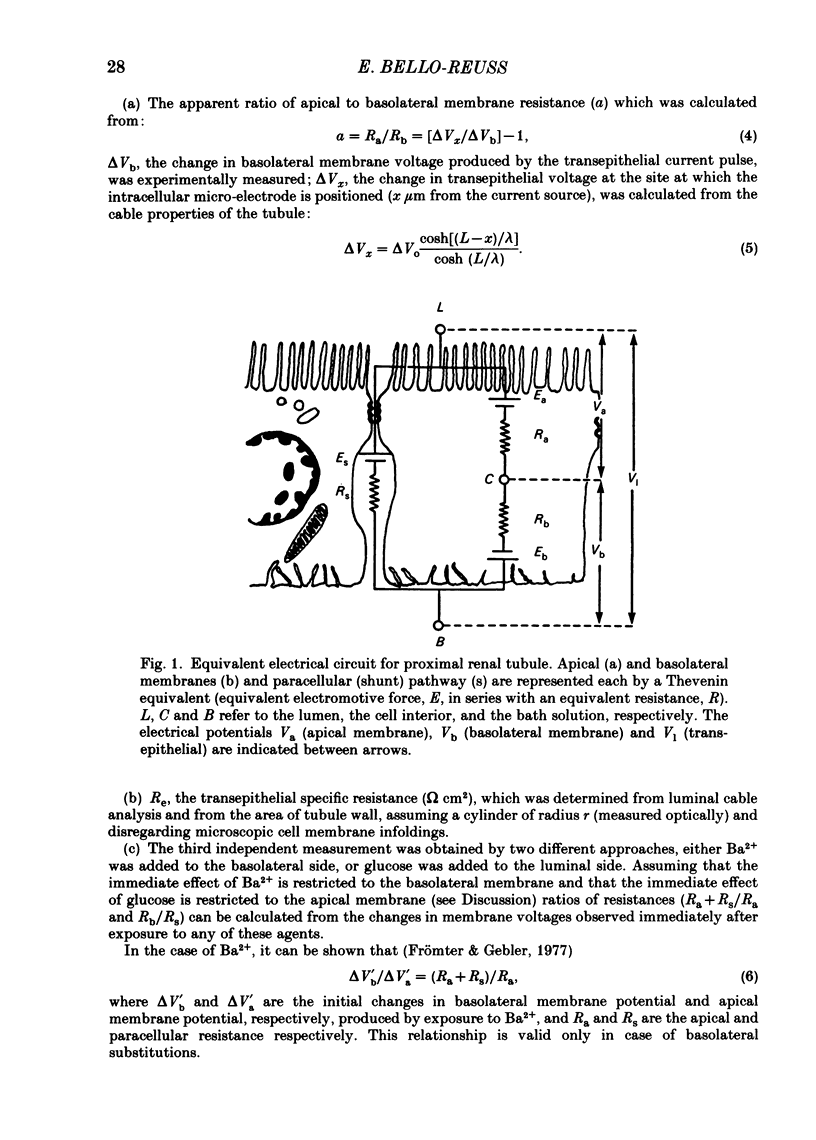
Abstract
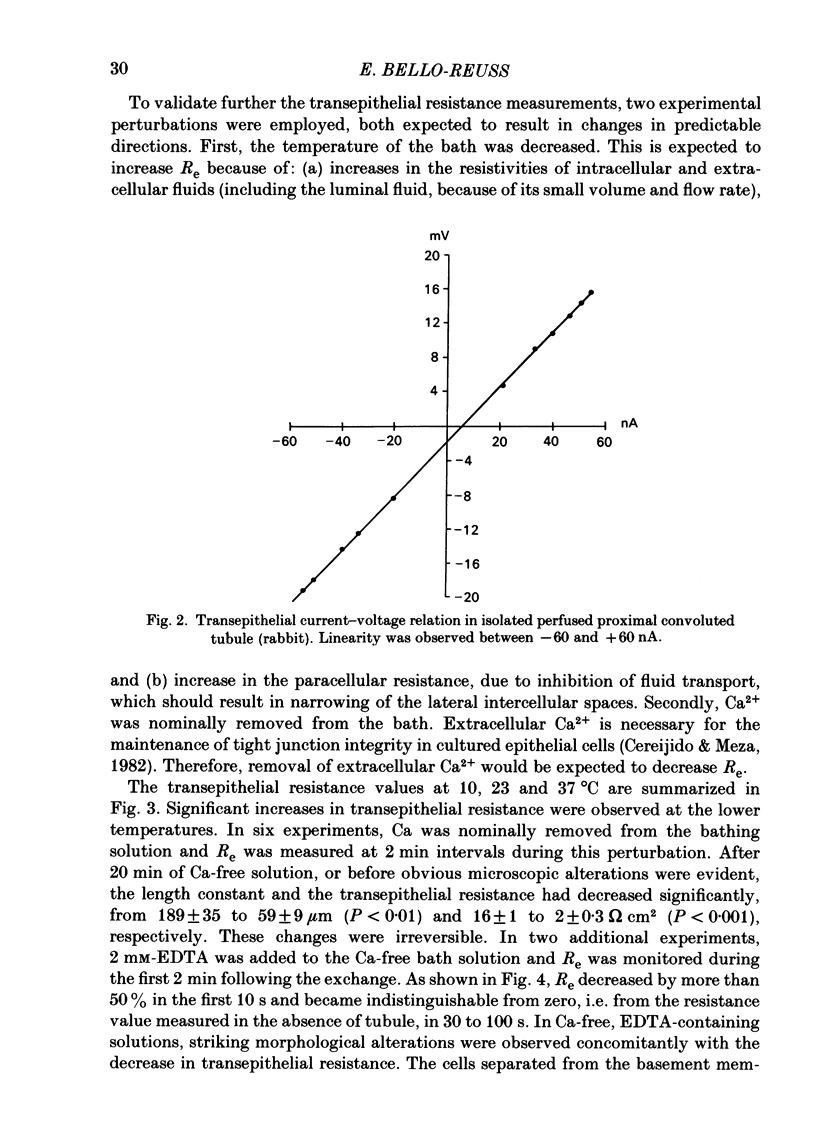
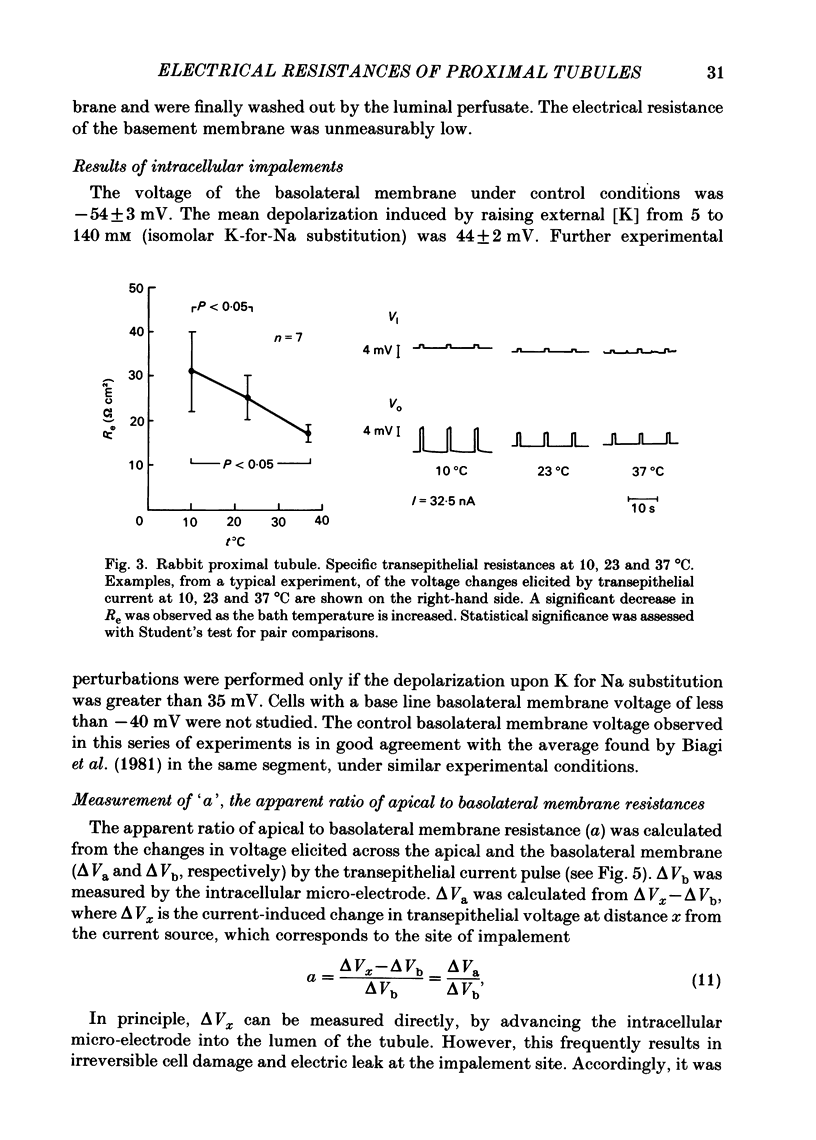
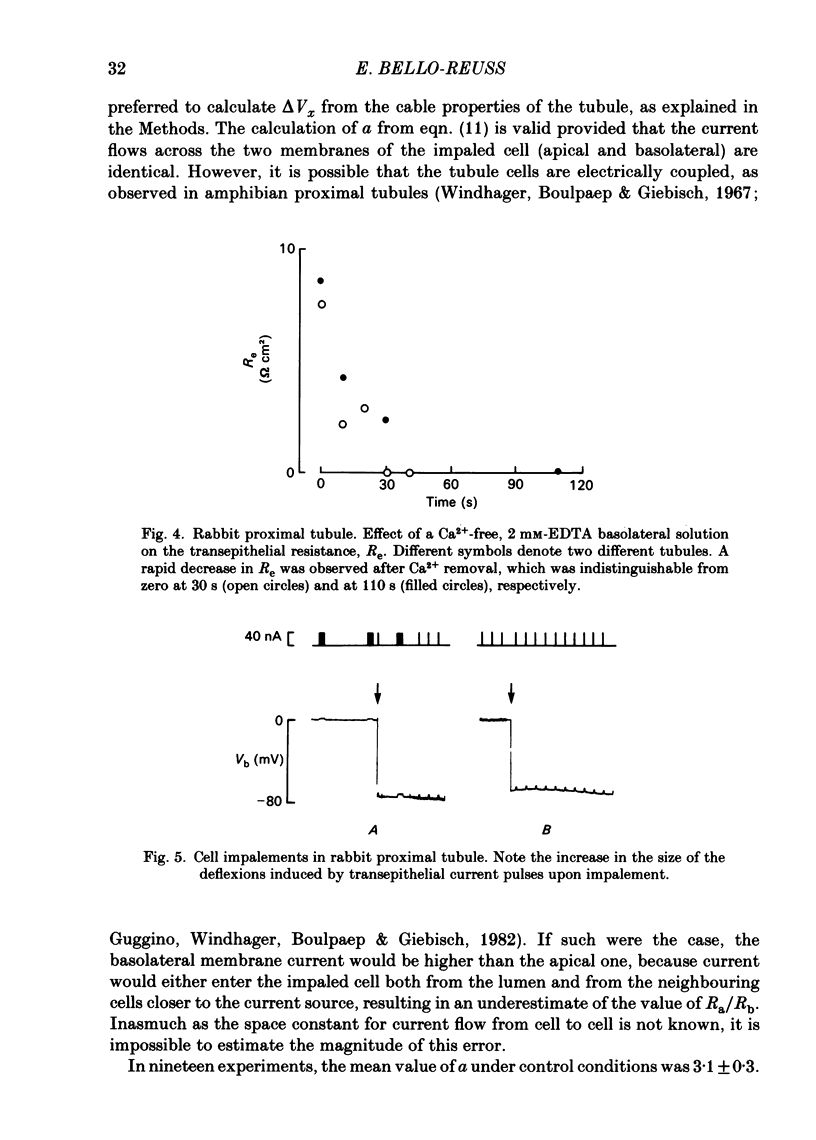
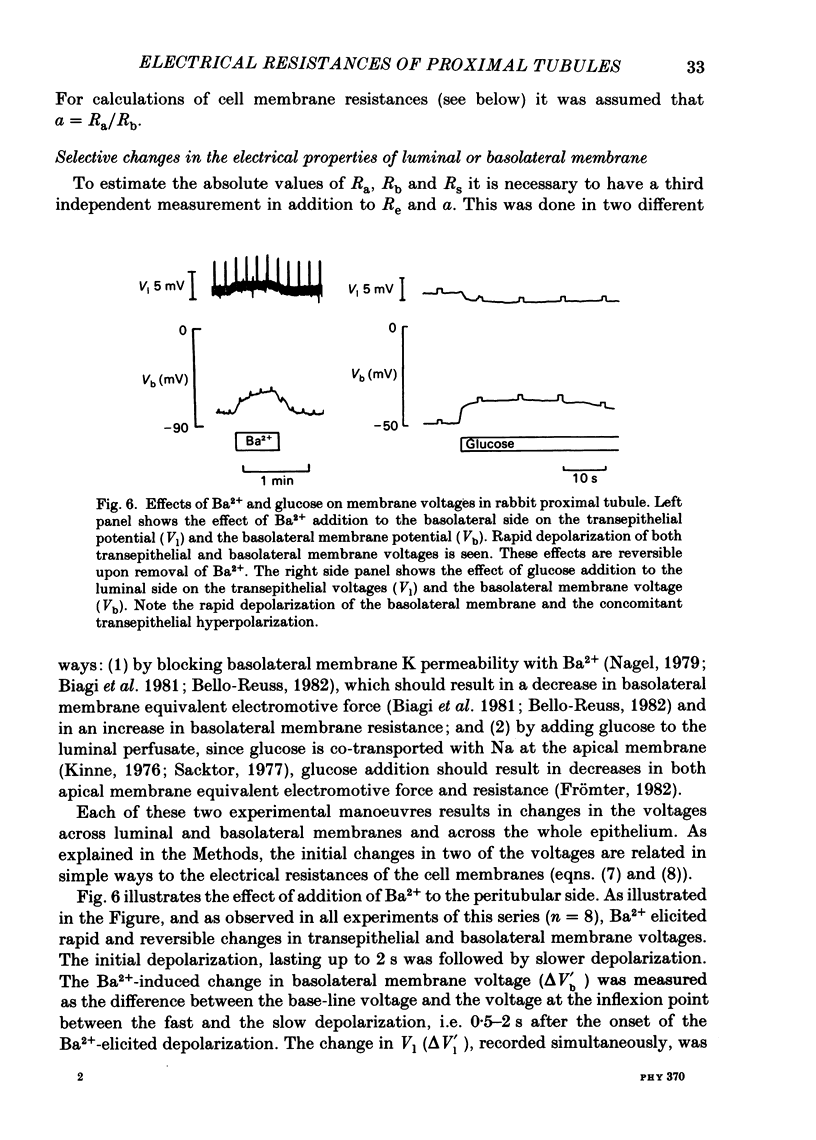
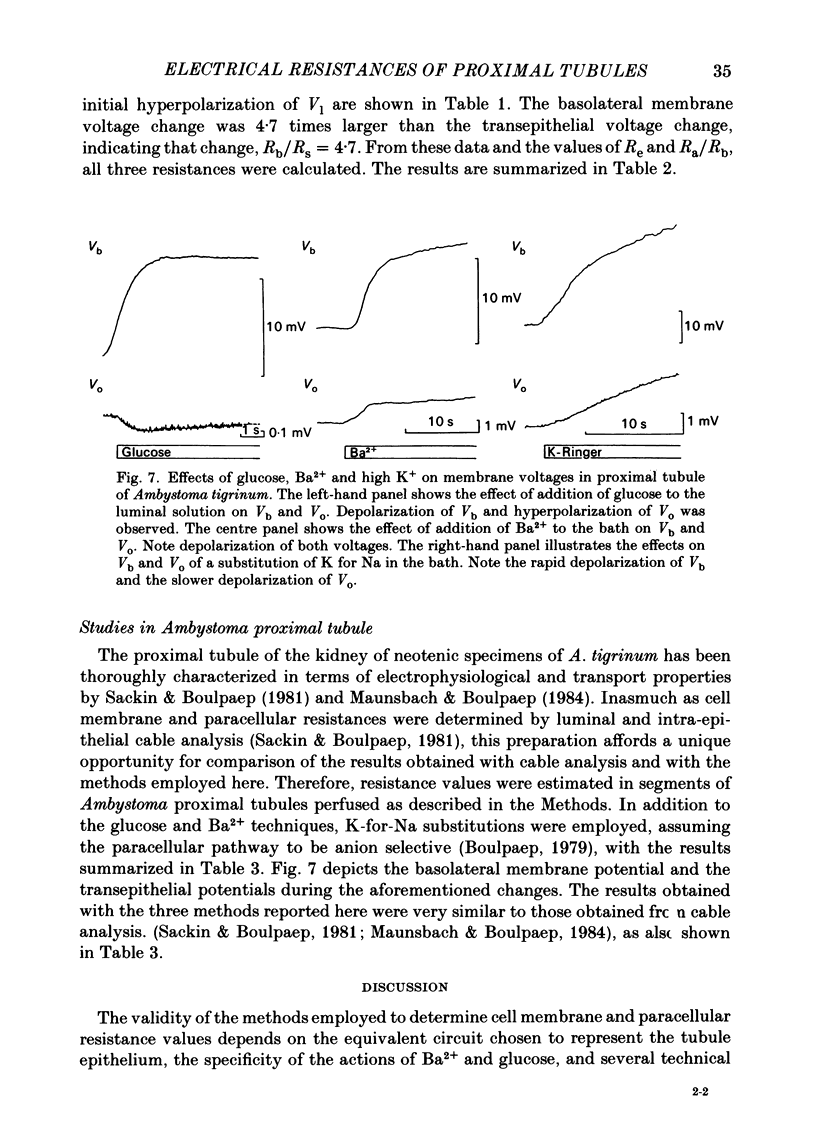
Transepithelial specific resistance (Re) was measured in isolated and perfused rabbit proximal convoluted tubules by cable analysis and intracellular micro-electrode techniques were used to calculate the electrical resistances of the cell membranes and of the paracellular pathway. Re was 16 +/- 2 omega cm2 and the space constant was 130 +/- 14 micron, n = 29. Re was significantly increased by a decrease in temperature from 37 to 10 degrees C, and was practically abolished by nominal removal of Ca2+ from the bathing solution (to 2.0 +/- 0.3 omega cm2, P less than 0.001, n = 6). The apparent ratio of cell membrane resistances (luminal to basolateral) was 3.1 +/- 0.3. The control values of apical and basolateral membrane resistances (Ra and Rb) were calculated from the values of (1) Re, (2) the apparent ratio of cell membrane resistances, and (3) the effects of addition of either Ba2+ (1 mM) to the bath solution or glucose (8 mM) to the perfusate on basolateral and apical membrane voltages (assuming that the initial effects of Ba2+ and glucose are restricted to the ipsilateral membrane). Control values of Ra (omega cm2 of epithelium) were 249 +/- 68 (Ba2+ method) and 227 +/- 42 (glucose method). Values of Rb were 70 +/- 11; and 66 +/- 12 respectively. The low paracellular resistance values obtained with the Ba2+ and glucose methods, respectively, 17 +/- 5 and 15 +/- 1 omega cm2, explain the low transepithelial resistance. The use of the Ba2+ and glucose methods provides alternatives to cell cable determinations for the calculation of cell membrane resistances. Cell membrane and shunt resistances measured by the same methods in isolated perfused Ambystoma tigrinum proximal tubules (in omega cm2 of epithelium) were: Ra, 2650 +/- 180 (glucose method) and 2368 +/- 350 (Ba2+ method). Values of Rb were 665 +/- 99 (glucose method) and 701 +/- 124 (Ba2+ method). The paracellular resistance values were 58 +/- 11 (glucose method) and 84 +/- 12 (Ba2+ method). These results are in good agreement with previously reported values obtained by intracellular cable analysis (Maunsbach & Boulpaep, 1984).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A. Lack of effect of peritubular protein on passive NaCl transport in the rabbit proximal tubule. J Clin Invest. 1983 Feb;71(2):268–281. doi: 10.1172/JCI110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B., Kubota T., Sohtell M., Giebisch G. Intracellular potentials in rabbit proximal tubules perfused in vitro. Am J Physiol. 1981 Mar;240(3):F200–F210. doi: 10.1152/ajprenal.1981.240.3.F200. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Issaacson L., Grantham J., Orloff J. Electrical properties of isolated perfused rabbit renal tubules. Am J Physiol. 1968 Oct;215(4):788–794. doi: 10.1152/ajplegacy.1968.215.4.788. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Frömter E., Gebler B. Electrical properties of amphibian urinary bladder epithelia. III. The cell membrane resistances and the effect of amiloride. Pflugers Arch. 1977 Oct 19;371(1-2):99–108. doi: 10.1007/BF00580777. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Greger R. Coupled transport of Na+ and Cl- in the thick ascending limb of Henle's loop of rabbit nephron. Scand Audiol Suppl. 1981;14 (Suppl):1–15. [PubMed] [Google Scholar]
- Guggino W. B., Windhager E. E., Boulpaep E. L., Giebisch G. Cellular and paracellular resistances of the Necturus proximal tubule. J Membr Biol. 1982;67(2):143–154. doi: 10.1007/BF01868657. [DOI] [PubMed] [Google Scholar]
- Helman S. I. Determination of electrical resistance of the isolated cortical collecting tubule and its possible anatomical location. Yale J Biol Med. 1972 Jun-Aug;45(3-4):339–345. [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Grantham J. J., Burg M. B. Effect of vasopressin on electrical resistance of renal cortical collecting tubules. Am J Physiol. 1971 Jun;220(6):1825–1832. doi: 10.1152/ajplegacy.1971.220.6.1825. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Lapointe J. Y., Laprade R., Cardinal J. Transepithelial and cell membrane electrical resistances of the rabbit proximal convoluted tubule. Am J Physiol. 1984 Oct;247(4 Pt 2):F637–F649. doi: 10.1152/ajprenal.1984.247.4.F637. [DOI] [PubMed] [Google Scholar]
- Lutz M. D., Cardinal J., Burg M. B. Electrical resistance of renal proximal tubule perfused in vitro. Am J Physiol. 1973 Sep;225(3):729–734. doi: 10.1152/ajplegacy.1973.225.3.729. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B., Boulpaep E. L. Quantitative ultrastructure and functional correlates in proximal tubule of Ambystoma and Necturus. Am J Physiol. 1984 May;246(5 Pt 2):F710–F724. doi: 10.1152/ajprenal.1984.246.5.F710. [DOI] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Sackin H., Boulpaep E. L. Isolated perfused salamander proximal tubule: methods, electrophysiology, and transport. Am J Physiol. 1981 Jul;241(1):F39–F52. doi: 10.1152/ajprenal.1981.241.1.F39. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]


