Abstract
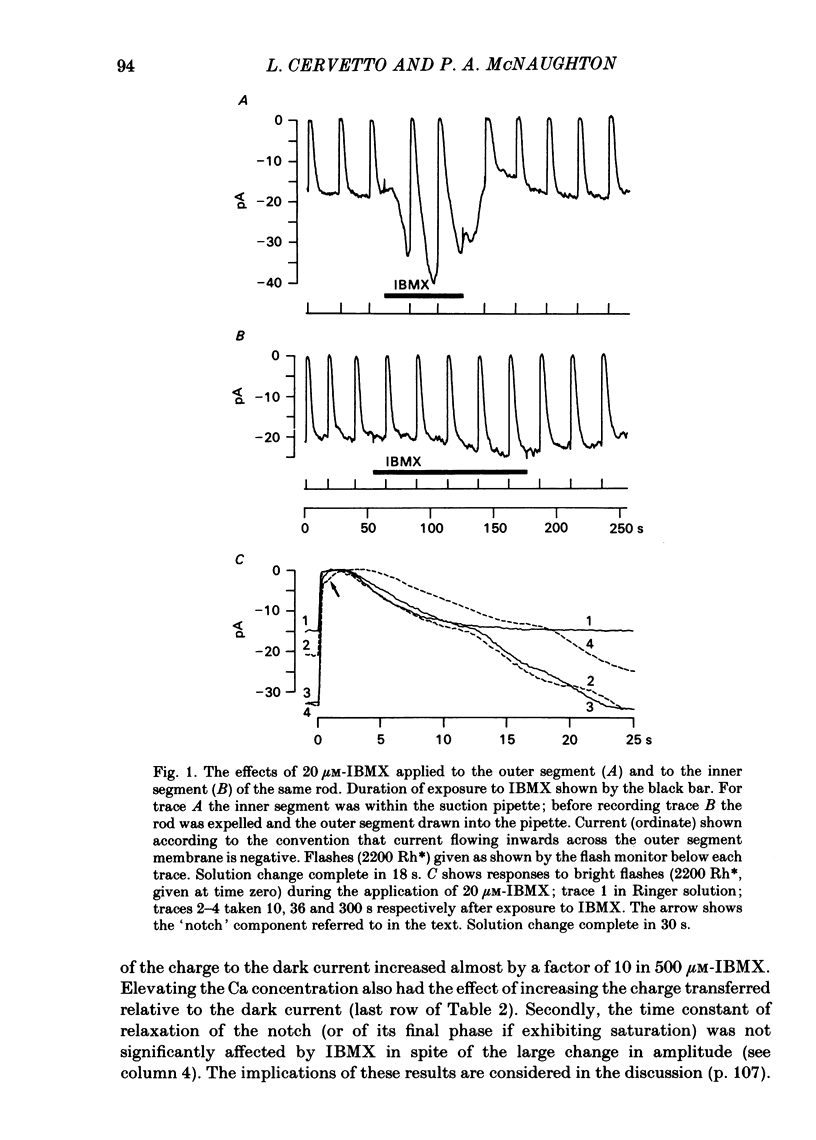
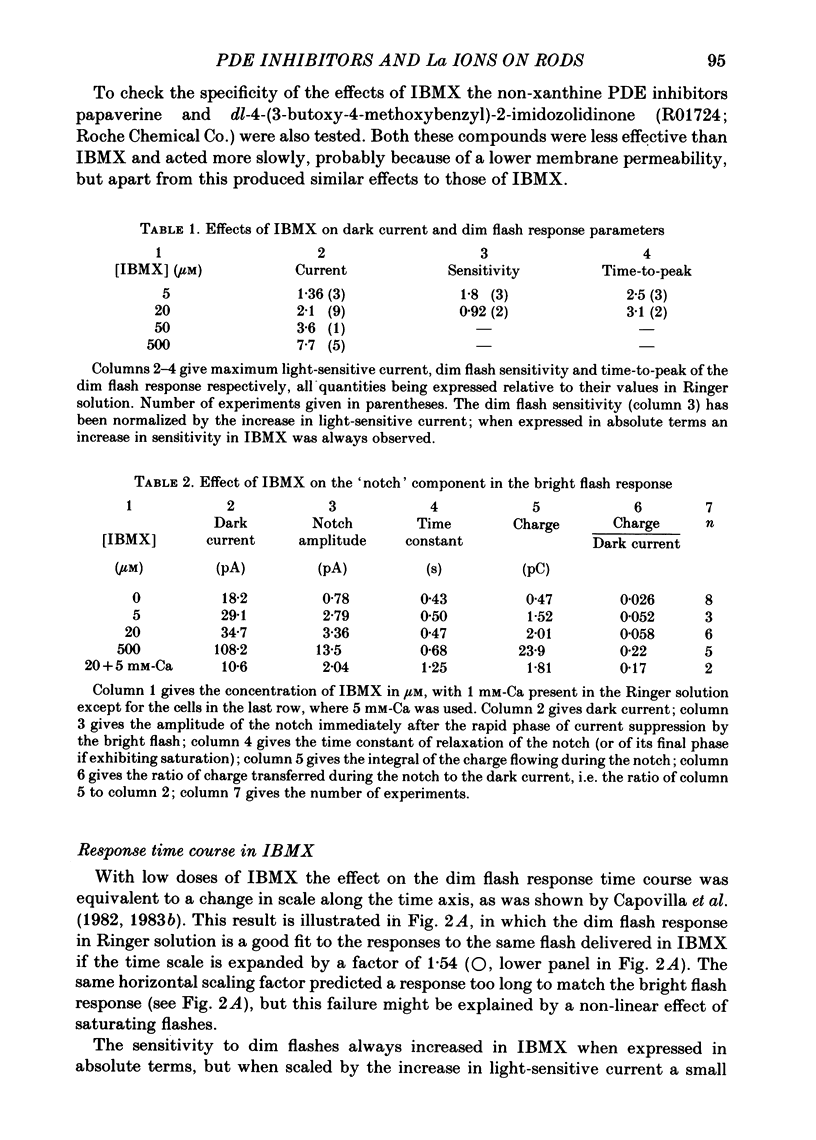
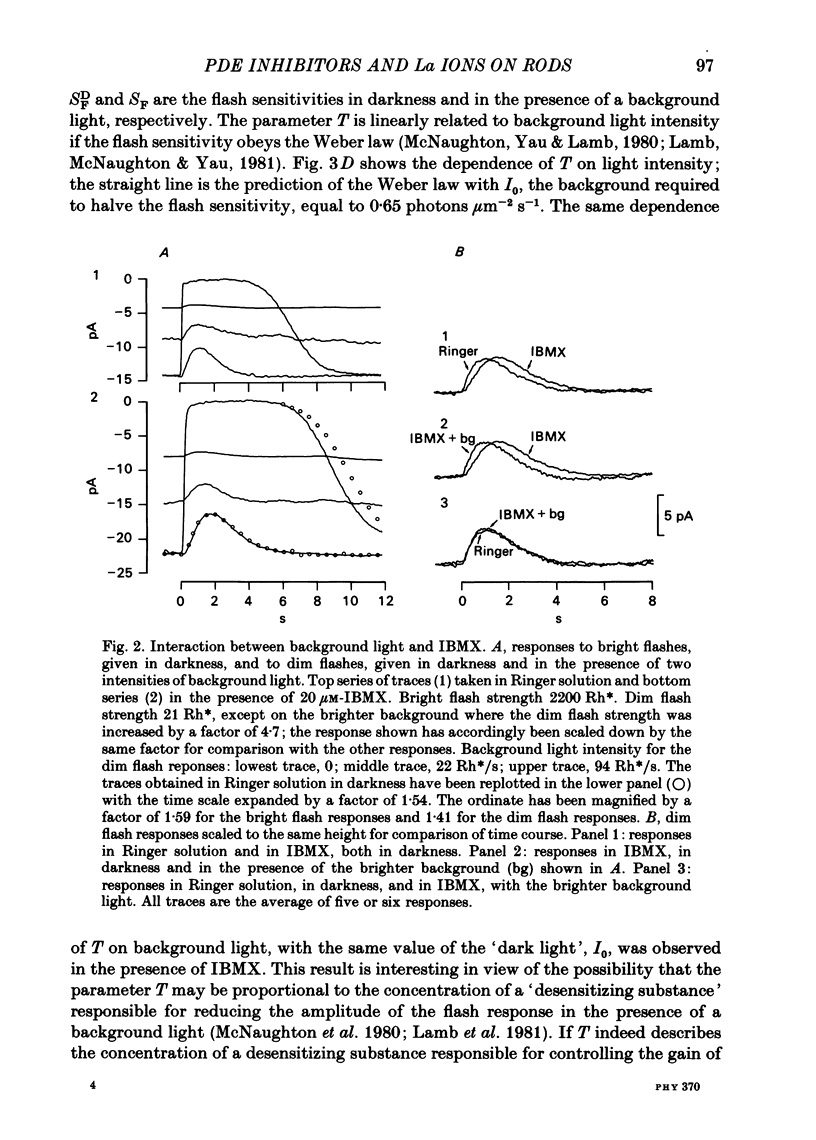
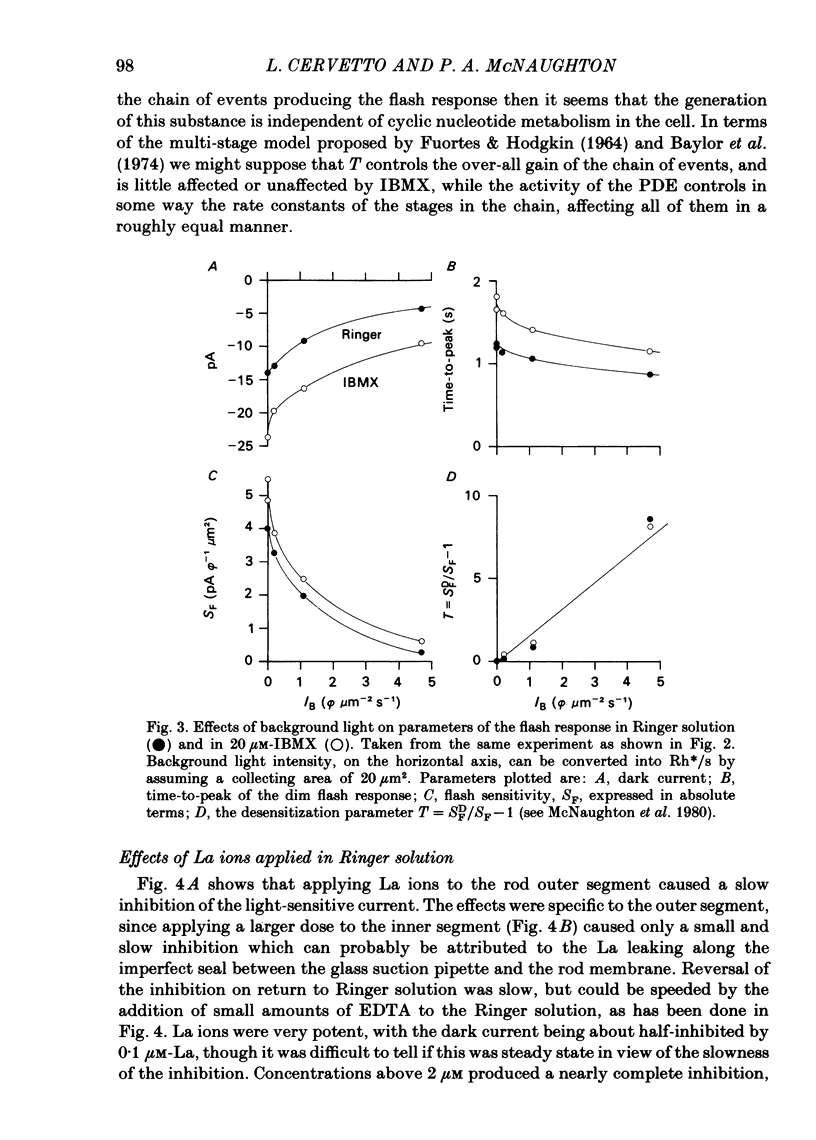
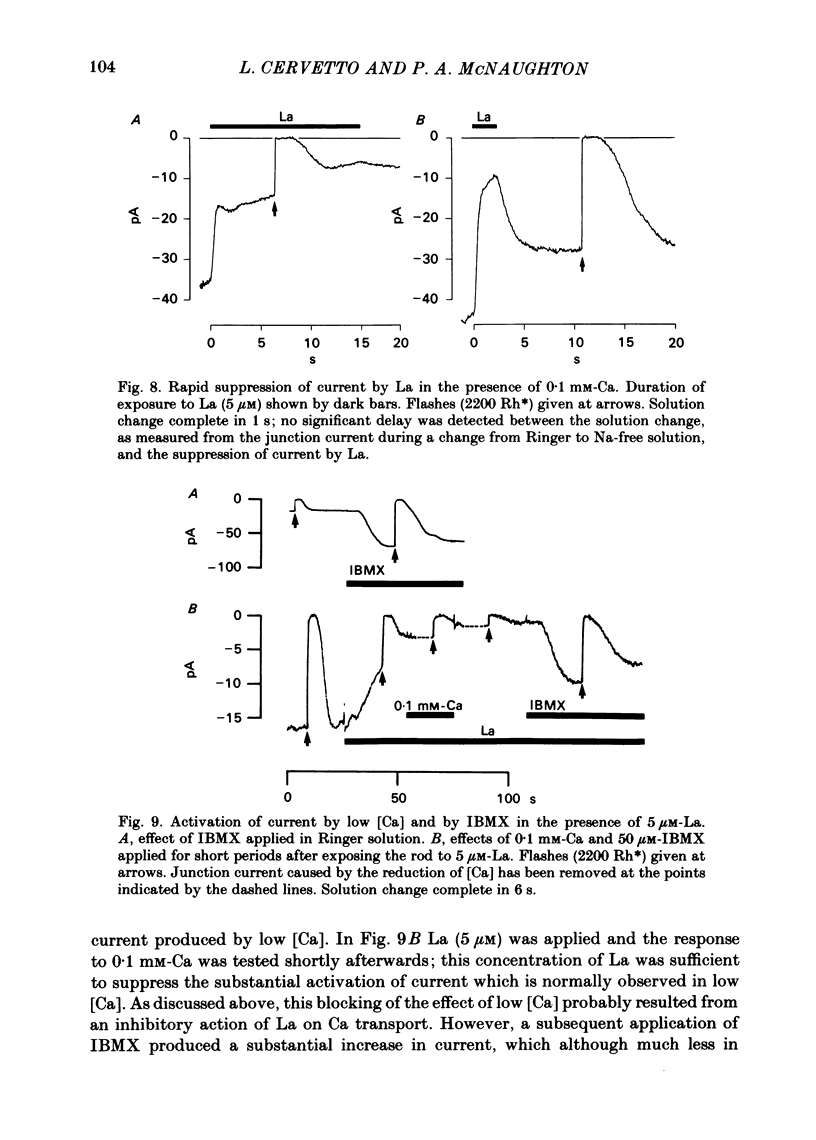
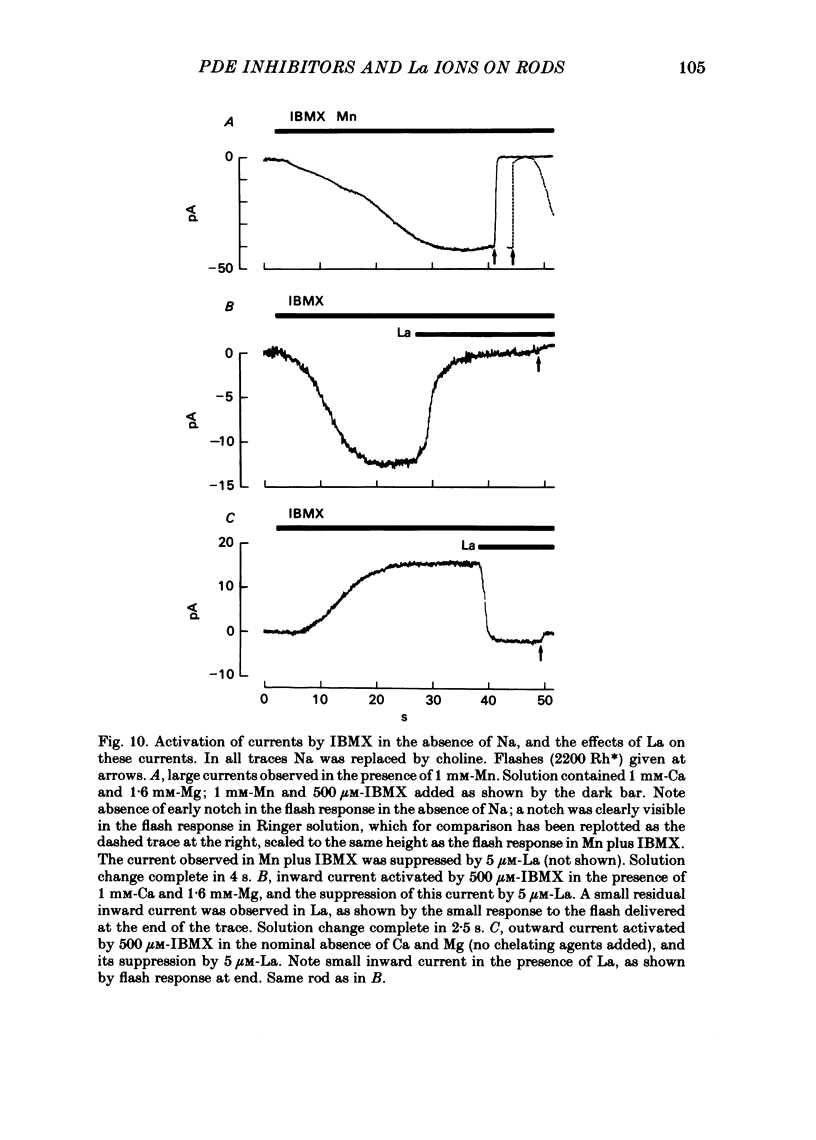
The light-sensitive current of isolated toad rods was recorded using the method of Yau, McNaughton & Hodgkin (1981) and the effects of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) and of La ions were examined. IBMX caused an increase in the light-sensitive current and a prolongation of the time course of the response. A small inward current which may reflect the operation of an Na-Ca exchange pump was also increased in IBMX. With low doses of IBMX the time course of the dim flash response could be mapped onto that in Ringer solution by a linear transformation of the time scale. Light adaptation had opposite effects to those of IBMX on the time course of the dim flash response, and a steady background light could exactly neutralize the effects of IBMX on the time course. Light adaptation had the additional effect of strongly reducing the amplitude of the dim flash response. La ions caused a rapid inhibition (t1/2 less than 1 s) followed by slow inhibition (t1/2 approximately equal to 30 s) of the light-sensitive current. In low [Ca] the rapid inhibition became more prominent, perhaps because of a competition between La and Ca for a blocking site near the light-sensitive channel. The time constants of the falling phases of responses to both bright and dim flashes were slowed by La. The dim flash response could be fitted by a model in which a single time constant in the chain underlying the flash response is slowed by La. La reduced the rate of activation of light-sensitive current in response to a reduction of external [Ca]. A concentration of La sufficient to block the activation of current in low [Ca] did not prevent the activation of current in IBMX. Light-sensitive currents carried by Mn, Ca or Mg in the absence of Na and in the presence of IBMX were inhibited by La. An outward current observed in the absence of permeable ions was inhibited by La. The effects of La on the time course of the response and on the rate of activation of current when [Ca] is reduced are consistent with an inhibition of the Ca pump. La ions also have a high affinity for the light-sensitive channel and can block current carried by another ion.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., McNaughton P. A. The influence of extracellular calcium binding on the calcium efflux from squid axons. J Physiol. 1978 Mar;276:127–150. doi: 10.1113/jphysiol.1978.sp012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Capovilla M., Caretta A., Cervetto L., Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol. 1983 Oct;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Antagonism between steady light and phosphodiesterase inhibitors on the kinetics of rod photoresponses. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6698–6702. doi: 10.1073/pnas.79.21.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. The effect of phosphodiesterase inhibitors on the electrical activity of toad rods. J Physiol. 1983 Oct;343:277–294. doi: 10.1113/jphysiol.1983.sp014892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., Bownds M. D. Visual transduction in vertebrate photoreceptors. Annu Rev Neurosci. 1979;2:17–34. doi: 10.1146/annurev.ne.02.030179.000313. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A., Yau K. W., Lamb T. D. Spread of activation and desensitisation in rod outer segments. Nature. 1980 Jan 3;283(5742):85–87. doi: 10.1038/283085a0. [DOI] [PubMed] [Google Scholar]
- Torre V., Pasino E., Capovilla M., Cervetto L. Rod photoresponses in the absence of external sodium in retinae treated with phosphodiesterase inhibitors. Exp Brain Res. 1981;44(4):427–430. doi: 10.1007/BF00238835. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- van Breemen C., De Weer P. Lanthanum inhibition of 45Ca efflux from the squid giant axon. Nature. 1970 May 23;226(5247):760–761. doi: 10.1038/226760a0. [DOI] [PubMed] [Google Scholar]


