Key Points
Question
Is hearing loss (HL) associated with falls?
Findings
In this systematic review and meta-analysis of 27 studies and more than 5 million participants, HL was associated with a 51% greater cross-sectional odds and 17% greater longitudinal risk of falls compared with individuals without HL.
Meaning
HL may be a risk factor for falls; physicians and policymakers should be aware of the ramifications of hearing health on aging and explore the potential role of hearing intervention for fall prevention.
This systematic review and meta-analysis evaluates the evidence surrounding the impact of hearing loss on falls.
Abstract
Importance
Falls constitute a significant public health concern worldwide and have been associated with increased morbidity and mortality across all ages. Identifying potentially modifiable risk factors for falls is a key public health priority. Literature surrounding the association between hearing loss (HL) and falls remains inconclusive.
Objective
To conduct a systematic review and meta-analysis to comprehensively synthesize evidence surrounding the impact of HL on falls.
Data Sources
PubMed, Embase, and Cochrane Library from database inception through April 9, 2024.
Study Selection
Observational studies investigating the association between HL and falls were selected. Only studies reporting covariate-adjusted estimates were included to minimize confounding.
Data Extraction and Synthesis
Two independent reviewers evaluated studies for eligibility, extracted data, and assessed the risk of bias of included studies. Using a random-effects model, adjusted estimates were pooled in meta-analyses. Heterogeneity was evaluated using subgroup and sensitivity analyses, and publication bias was assessed.
Main Outcomes and Measures
The cross-sectional odds and longitudinal risk of falls among patients with HL compared with those without HL.
Results
A total of 5 071 935 participants were included from 27 studies; approximately 49.2% of participants were female, and 14 studies were conducted in Asia, 7 in North America, 3 in Europe, and 3 in Oceania, represented by Australia. Patients with HL exhibited an increased cross-sectional odds of falls (odds ratio, 1.51; 95% CI, 1.37-1.67; I2 = 64%) and longitudinal risk of falls (risk ratio, 1.17; 95% CI, 1.06-1.29; I2 = 69%) than those without HL. Further stratification by self-reported or validated hearing assessments, fall reporting duration, continent, community-dwelling adults, and studies adjusting for other sensory deficits identified as fall risk factors by the World Falls Guideline did not change significance. These results remained robust to sensitivity analyses, and publication bias was absent.
Conclusions and Relevance
This systematic review and meta-analysis found that overall, HL may be a risk factor for falls. With a rapidly aging global population, it is crucial to acknowledge the public health concerns surrounding falls and consider if HL could be a potentially modifiable risk factor. Nonetheless, further randomized clinical trials are needed to elucidate any benefit of treating HL on fall prevention.
Introduction
Falls represent a major global public health concern as well as a persistent risk to morbidity and mortality across all ages,1 ranking as one of the most prevalent causes of disability from injuries globally.2 The issue of population aging accentuates concerns about falls, particularly among older adults,3 as epidemiological research suggests that the global burden of falls is most pronounced in this demographic.1 Falls are defined as an unexpected event where an individual comes to rest on the ground, the floor, or a lower level.4 The majority of falls result from a combination of factors that increase an individual’s susceptibility to falling, along with acute mediating factors that create the opportunity for a fall.5
Hearing loss (HL) is a highly prevalent condition associated with aging and has emerged as a significant global health concern, particularly due to its link with cognitive impairment and dementia6 and its potential role as a modifiable risk factor for various age-related conditions.7,8 Presently, the relationship between HL and falls is inconclusive. While various studies suggest that HL may be an independent risk factor for falls,9,10 others contend that it may not significantly influence the propensity to fall or that the risk of falling may be mediated through confounding factors, such as visual impairment, vestibulopathy, and cognitive decline.11,12 Therefore, a thorough review and synthesis of current evidence may add clarity to preexisting literature.
The objective of this study is to systematically review the preexisting literature and pool their data in a meta-analysis to determine the association of HL with cross-sectional odds and longitudinal risk of falls. Given the considerable public health concern surrounding the morbidity and mortality associated with falls,1 establishing HL as a potentially modifiable risk factor may further emphasize the importance of addressing hearing health. We hypothesize that HL may be an independent risk factor for falls, and a cohesive summary and analysis of existing literature on this subject is both clinically relevant and timely.
Methods
This systematic review and meta-analysis was registered on PROSPERO (CRD42024534255). This research adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.13
Data Sources and Searches
We conducted a comprehensive search of 3 databases (PubMed, Embase, and Cochrane Library) from database inception to April 9, 2024, using search terms pertaining to HL and falls and their relevant synonyms. The full search strategy is provided in eTable 1 in Supplement 1.
Study Selection
The study selection process by titles and abstracts, followed by full-text review, was conducted by 2 of us (B.S.Y.Y. and V.Y.J.T.), with disagreements resolved through the consensus of another independent author (J.H.N., J.Z.T., or B.L.H.S.). Based on the Population, Intervention, Comparators, Outcomes framework,14 we selected studies that met the following inclusion criteria: (1) adults 18 years older with HL; (2) HL identified using validated clinical methods, such as pure-tone audiometry and International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes, or through self-reported measures such as questionnaires; (3) falls identified either through self-reported measures or hospitalization records; (4) the outcomes of interest being the cross-sectional odds and longitudinal risk of falls among patients with HL compared with those without HL. We included observational studies published as full-length English-language articles in peer-reviewed journals, reporting covariate-adjusted estimates to minimize confounding.
We excluded studies that did not meet the inclusion criteria, as well as duplicates, case reports, case series, reviews, meta-analyses, letters, conference abstracts, pediatric studies, animal studies, non–English-language studies, studies with incomplete data, and studies lacking a clear definition of HL. In instances where multiple studies satisfying our inclusion criteria used data from the same overarching cohort, we included the study with the largest sample size.
Data Extraction
The data of interest from the included articles were extracted by 2 of us (B.S.Y.Y. and V.Y.J.T.) using a structured template. The details of data extraction were verified by another independent author (J.H.N., J.Z.T., or B.L.H.S.). This includes the information pertaining to study design, baseline population characteristics, method of measuring hearing function, HL criteria, method of evaluating for falls, duration for reporting falls, study outcomes, type of statistical reporting ratio used, covariate-adjusted ratios and their respective 95% CIs, and the list of confounders accounted for.
Data Synthesis and Statistical Analysis
All statistical analyses were performed using R Studio version 4.0.3 (Posit PBC) using the meta and metafor packages.15 We used a random-effects model to pool covariate-adjusted ratios to account for anticipated clinical heterogeneity.16 Whenever feasible, generalized linear mixed models with logit-transformed data were used to prevent errors associated with the double-arcsine transformation. Thompson I2 values and the Cochran Q test were adopted to assess for heterogeneity, where an I2 value of 25%, 50%, and 75% represented low, moderate, and high degrees of heterogeneity, respectively, and a τ2 P value of .10 or less was considered significant for heterogeneity.17,18 To directly quantify heterogeneity, τ2 was concurrently estimated, as I2 is known to artificially increase with larger sample sizes even when heterogeneity remains constant.17 Unless otherwise specified, a 2-sided P value less than .05 was considered statistically significant.
Additional prespecified subgroup analyses and sensitivity analyses were conducted to identify potential sources of heterogeneity when sufficient information was available. The subgroup analyses included self-reported vs validated clinical methods of determining HL, the duration for reporting falls, and the geographical continent of study. We also pooled studies that exclusively included community-dwelling individuals as well as those that specifically adjusted for other sensory deficits established as risk factors for falls by the World Falls Guideline,19 including vision impairment and dizziness or vestibulopathy.
Further leave-1-out influence analyses and cumulative analyses were conducted to examine the effect of individual studies on the overall findings and ascertain the stability of published data over time, respectively. Whenever a minimum of 10 studies were pooled per outcome, meta-regression was performed to account for other potential confounding variables, including age, gender, and follow-up duration. We evaluated for publication bias qualitatively by visually inspecting for funnel plot asymmetry and quantitatively using the Egger regression test.20,21 The trim-and-fill method was used to input potentially missing studies.22
Quality Assessment
The risk of bias in observational studies was appraised by 2 independent authors (B.S.Y.Y. and V.Y.J.T.) using the Newcastle-Ottawa Scale (NOS), with differences resolved through the consensus of another independent author (J.H.N., J.Z.T., and B.L.H.S.).23 Separate NOS scales were used for cross-sectional, cohort, and case-control studies. The studies were categorized as low, moderate, or high risk of bias based on their scores of 8 or more, 5 to 7, and less than 5 points, respectively. The overall quality of pooled evidence at the outcome level was assessed using the Grading of Recommendations, Assessment, Development and Evaluations framework.24,25
Results
The initial literature search of PubMed, Embase, and Cochrane Library found 3372 studies, of which 270 duplicates were removed. The titles and abstracts of 3102 unique articles were screened, of which 3025 were excluded. Subsequently, the full texts of 77 remaining articles were assessed for eligibility, and 48 articles were further excluded. We included 27 articles comprising 5 071 935 participants. The PRISMA flowchart of study inclusion can be found in Figure 1.
Figure 1. PRISMA Flowchart.
Study Characteristics
Approximately 49.2% of participants were female. Geographically, 14 studies were conducted in Asia,11,26,27,28,29,30,31,32,33,34,35,36,37 7 in North America,10,12,38,39,40,41 3 in Europe,42,43,44 and 3 in Oceania, represented by Australia.45,46,47 There were 15 cross-sectional studies, 11 cohort studies, and 1 case-control study. All studies included community-dwelling adults except those by Shen et al33 and Tiase et al,40 which included institutionalized adults. eTable 2 in Supplement 1 presents the key characteristics extracted from the included studies. Based on the NOS, 3 studies had a low risk of bias, 24 studies had a moderate risk of bias, and none had a high risk of bias (eTables 3 to 5 in Supplement 1). The overall quality of evidence per outcome according to the Grading of Recommendations, Assessment, Development and Evaluations framework was low (eTable 6 in Supplement 1).
Assessment of Hearing Function and Ascertainment of Falls
The hearing function of participants was measured using validated clinical methods, such as pure-tone audiometry in 9 studies, while 18 studies used self-reported measures to determine HL, such as patient questionnaires. Separately, the outcome of prevalent falls was investigated in 20 studies, of which 18 relied on self-reported questionnaires, while 2 studies (Shen et al33 and Tiase et al40) used an item of the Morse Fall Scale. The outcome of incident falls was examined by 9 studies, of which those by Deal et al10 and Tonelli et al48 used ICD-9 and ICD-10 codes to identify falls, while the remaining 7 studies used self-reported measures. eTable 2 in Supplement 1 outlines the methods of measuring hearing function and identifying falls in greater detail.
Longitudinal Association of HL and Incident Falls
HL was associated with a 17% greater longitudinal risk of falls compared with individuals without HL (risk ratio, 1.17; 95% CI, 1.06-1.29; I2 = 69%; 9 studies) (Figure 2).10,11,32,36,37,42,45,46,48 Further meta-regression analyses outlined that age, but not gender and follow-up duration, was a significant moderator of the overall effect size (eTable 7 in Supplement 1). While age did not account for any additional heterogeneity, the pooled effect size increased by a factor of 0.29 (95% CI, 0.02-1.15) per year of increase in the mean age. The leave-1-out influence analysis revealed that the exclusion of any single study did not affect the overall significance of the pooled effect size (eFigure 1 in Supplement 1), and cumulative meta-analysis outlined the stability of the pooled result over time (eFigure 2 in Supplement 1). While visual inspection outlined possible funnel plot asymmetry, there was no evidence of publication bias based on the Egger regression test (intercept = −1.64; P = .14) (eFigures 3 and 4 and eTable 7 in Supplement 1). Additional trim-and-fill analysis did not input any potentially missing studies (eTable 8 in Supplement 1).
Figure 2. Forest Plot of the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss.

RR indicates risk ratio.
Cross-Sectional Association of HL and Prevalent Falls
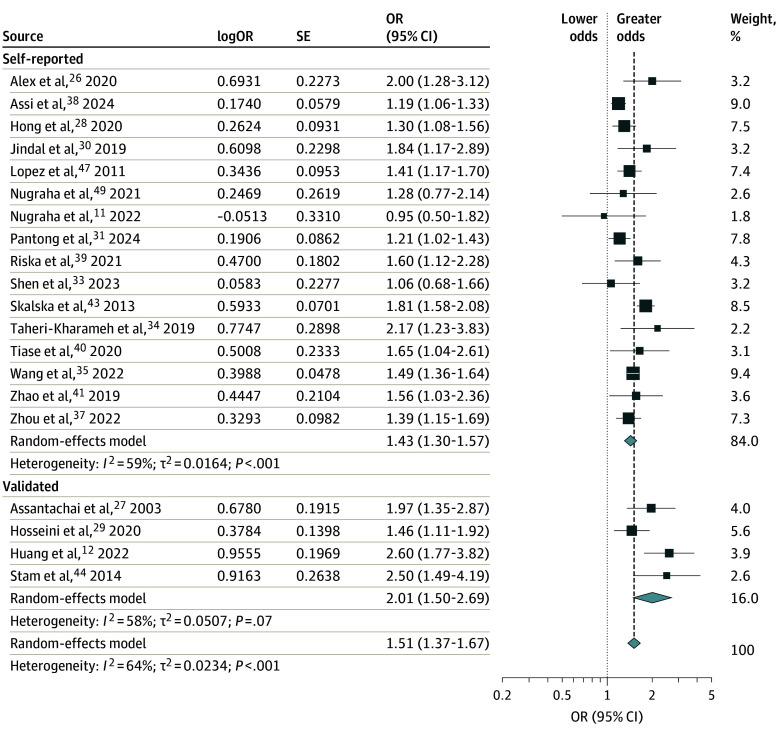
HL was associated with a 51% greater cross-sectional odds of falls compared with individuals without HL (odds ratio [OR], 1.51; 95% CI, 1.37-1.67; I2 = 64%; 20 studies) (Figure 3).11,12,26,27,28,29,30,31,33,34,35,38,39,40,43,44,47,49 The cross-sectional odds of falls were greater among studies using validated measures of hearing assessment (OR, 2.01; 95% CI, 1.50-2.69; I2 = 58%; 4 studies) than studies that assessed hearing using self-reported measures (OR, 1.43; 95% CI, 1.30-1.57; I2 = 56%; 16 studies) (Figure 3). The results also remained robust to other subgroup analyses of duration for reporting falls and geographical continent (eFigures 5 and 6 in Supplement 1). HL was also associated with a greater cross-sectional odds of falls among exclusively community-dwelling adults (eFigure 7 in Supplement 1).
Figure 3. Forest Plot of the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Stratified by Self-Reported vs Validated Methods of Hearing Testing.
OR indicates odds ratio.
Crucially, HL was still associated with an increased cross-sectional odds of falls in studies that corrected for vision impairment (OR, 1.47; 95% CI, 1.28-1.69; I2 = 37%; 11 studies) (Figure 4A)11,26,27,30,31,33,34,39,41,47,49 and dizziness or vestibulopathy as a confounder (OR, 1.77; 95% CI, 1.38-2.27; I2 = 0%; 3 studies) (Figure 4B).30,34,39 Importantly, no significant heterogeneity was observed among studies that exclusively adjusted for these sensory impairments.
Figure 4. Forest Plot of the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss Among Studies That Exclusively Corrected for Other Sensory Risk Factors for Falls.
OR indicates odds ratio.
Using meta-regression, age was identified as a significant effect moderator of the effect size, accounting for 100% of heterogeneity. The pooled OR increased by a factor of 0.13 (95% CI, 0.22-0.74) per year of increase in the mean age. However, gender did not affect the overall observed effect size (eTable 7 in Supplement 1).
Further leave-1-out influence analysis outlined that the omission of any study did not alter the overall significance of the pooled effect size (eFigure 8 in Supplement 1), and cumulative meta-analysis demonstrated stability of the pooled result and narrowing of the 95% CI range over time (eFigure 9 in Supplement 1). There was no funnel plot asymmetry detected by visual inspection or Egger regression test (intercept = 0.54; P = .60) and thus no evidence of publication bias (eFigures 10 and 11 and eTable 8 in Supplement 1). While the trim-and-fill analysis added 2 additional studies, there were no changes to the significance of the overall effect size (OR, 1.46; 95% CI, 1.32-1.61; I2 = 69%) (eTable 8 in Supplement 1).
Discussion
This systematic review and meta-analysis of 27 studies and 5 071 935 participants found that HL may be associated with increased cross-sectional odds and longitudinal risk of falls compared with adults without HL. HL may be an independent risk factor for falls after adjusting for other sensory deficits identified as fall risk factors by the World Falls Guideline, including visual impairment and dizziness or vestibulopathy. These results remained robust to a variety of subgroup and sensitivity analyses, and no evidence of publication bias was observed. The present study expands on preexisting literature on the relationship between HL and falls and underscores the importance of recognizing HL as a risk factor for falls among other more well-established risk factors.50
To our knowledge, this comprehensive evidence-based summary of the literature on the association between HL and falls to date is the first meta-analysis to aggregate the longitudinal relationship between HL and falls. The cross-sectional association between HL and falls was previously pooled in a meta-analysis of 12 studies by Jiam et al51 in 2016, who found that older adults with HL had 2.39-fold greater odds of falling than older adults with normal hearing. However, the authors acknowledged that their findings were limited by a scarcity of well-adjusted studies, significant heterogeneity, and indications of publication bias. The findings of our study expand on the aforementioned literature by including more than double the number of studies and addressing key limitations identified in previous research. While Jiam et al51 combined both unadjusted and adjusted studies, we focused solely on studies that provided adjusted estimates to minimize the influence of confounding variables, thereby increasing the reliability of our findings.52 Furthermore, our study observed reduced heterogeneity in the pooled associations, likely due to the use of covariate-adjusted estimates. Additionally, our study examined the association of HL with the odds of injury from falls and the longitudinal risk of falls, a comprehensive approach not feasible in the earlier meta-analysis due to limited available literature. With greater availability of literature, further subgroup and sensitivity analyses were conducted, enhancing the robustness of our findings. We also did not identify any publication bias, a key limitation highlighted in the previous meta-analysis. Crucially, we found that the association between HL and falls remained significant even when accounting for concurrent risk factors for falls, such as vision impairment and vestibulopathy, addressing a significant limitation acknowledged in prior research.
Compared with other meta-analyses of observational studies examining the association between sensory impairments and falls, our findings suggest that the magnitude of fall risk associated with HL may be comparable with other well-established sensory-related risk factors, such as vision loss and vestibulopathy. For example, a meta-analysis of observational studies by Deandrea et al5 suggested that vision impairment and vestibulopathy increased the odds of falls by 35% and 80%, respectively. While making indirect comparisons between our findings and the results of other meta-analyses should be approached with caution due to potential differences in study variables, our results suggest that HL may warrant attention comparable with that given to other well-established sensory-related risk factors for falls.
There are various plausible mechanisms that may explain the observed association between HL and falls. First, patients with HL may experience increased demands in listening and communication, potentially reducing the attentional resources crucial for balance control.53 The maintenance of balance relies on the brain’s ability to integrate sensorimotor inputs from various parts of the body, which requires intricate neural processing.54 Various magnetic resonance imaging studies have demonstrated that individuals with imaging-detected cerebral atrophy experience greater difficulties with balance problems compared with those without structural cerebral changes.55 Similarly, HL has been linked to accelerated brain atrophy,56 drawing a potential structural link between HL and postural imbalance, which may increase the risk of falling.
Second, HL may also directly restrict an individual’s access to auditory information essential for spatial awareness and maintaining postural balance.57 Agmon et al58 conducted a systematic review that found that HL may be associated with poorer postural control in older adults. These findings were supported by a meta-analysis by Foster et al,59 which further highlighted that older adults with moderate to severe HL may exhibit more postural instability compared with those with normal or mild HL. Importantly, their findings align with previous studies suggesting that HL contributes to slower gait speed,60 increased stride length variability,61 poorer postural control,62 and reduced walking endurance.63 These auditory cues may serve as acoustic landmarks that aid in spatial awareness and perception, which may influence postural stability and reactive balance.64,65 This association between HL and balance difficulties has been linked to an increased risk of falls in both younger and older adults.62 These observations suggest that postural imbalance may mediate the relationship between HL and falls.
Third, other shared risk factors and pathophysiological mechanisms between HL and falls may also shed light on their correlation. For example, cardiovascular disease and diabetes have been linked with both HL and falls.66,67 Individuals with cardiovascular disease and diabetes may develop microangiopathic changes in the cochlea, decreasing microcirculation and potentially causing HL.66,68,69,70 Similarly, diabetic microangiopathy may also cause peripheral neuropathy and a loss of proprioception, which may affect somatosensory components of posture control.71 Hence, these shared risk factors may draw an indirect link between HL and falls. Nonetheless, the use of covariate-adjusted estimates robustly accounted for a variety of these shared risk factors.
It is crucial to acknowledge that there are myriad intrinsic and extrinsic risk factors that may increase an individual’s propensity to fall.72 The association between HL and falls remained significant in studies that had preadjusted for confounders, suggesting that HL may be an independent risk factor for falls. Various earlier studies have suggested that HL and falls may be mediated by cognitive decline.73,74 HL is a well-established factor for cognitive impairment and dementia,74 and patients with cognitive deficits may have fewer cognitive reserves for executive function and other activities that require neural integration.75 Separately, considering the close anatomical proximity of both vestibular and auditory systems,76 previous studies have suggested that certain factors that can cause both dizziness and HL, such as age-related degeneration,77 ototoxic medications78 or otologic conditions like labyrinthitis,76 may be partly responsible for the observed association between HL and falls. However, HL was still significantly associated with an increased odds of falling in studies that preadjusted for dizziness or vestibulopathy. Importantly, no heterogeneity was observed in this pooled association. This suggests that the mechanisms behind the association between HL and falls may extend beyond the effects of HL-related vestibular impairments. Nonetheless, future research may provide greater insights into the intricacies of the relationship between HL, vestibulopathy, and falls. Additionally, further studies may add value to the current literature by directly comparing the risk of falling between patients with isolated HL and those with other concomitant risk factors for falls.
We noted a higher odds of falling among patients with HL identified through validated methods of testing compared with those using self-reported questionnaires. We suspect that studies using self-reported measures underestimate the true prevalence of HL because individuals often underestimate their hearing difficulties, attributing them to the natural aging process.79 Nonetheless, a significant association between HL and falls was observed in both subgroups, which underscores the need to take caution even among individuals with self-reported claims of HL.
Not surprisingly, meta-regression analysis outlined that age was a significant effect modifier of the association between HL and falls. We observed that an increase in the mean age of participants increased the odds and risk of falling. These observations are supported by previous epidemiological studies.80 Salari et al81 conducted a meta-analysis that identified the pooled global prevalence of falls in older adults to be 26.5%. The risk of falling in older adults may be elevated due to the decline of various neural components necessary for maintaining balance,82 the increased likelihood of multimorbidity, and the greater risk of polypharmacy.83 Moreover, poorer functional status in older adults may make it more challenging for them to navigate around environmental hazards, thereby increasing their susceptibility to falls.84 Cumulatively, these risk factors contribute to an increase in fall risk with age. Nonetheless, systematically reviewed studies have also shown that HL may be associated with falls in younger adults.
The findings of this study carry significant implications for advancing our understanding of the impact of HL on aging. HL is highly prevalent,6 and its effects are potentially treatable with hearing rehabilitative devices, which remain underused.85 Although previous studies have proposed potential cognitive and mortality benefits of hearing restorative devices,7,86 current evidence on the role of hearing intervention in preventing falls is inconclusive among observational studies.87,88 Additionally, more randomized clinical trials are needed to examine the benefits of treating HL in the context of aging.8,89 However, conducting randomized studies of this nature may be challenging due to ethical and administrative difficulties in justifying a control group that does not receive any hearing intervention. Hence, this underscores the importance of findings from observational meta-analyses.
The growing body of evidence surrounding the adverse effects of HL in older adults should prompt physicians and policymakers to adopt a more vigilant approach to hearing health. Considering the dire consequences of falls,1 identifying HL as a potential risk factor for falls may carry significant implications in public health.90,91 Currently, there is a lack of consensus among recommendation articles regarding the classification of HL as a risk factor for falls. While the World Falls Guideline recommends examining HL as part of a multifactorial falls risk assessment19 as well as referring individuals with HL to a specialist when appropriate, not all concur.92 Crucially, the findings of this study suggest that it may be useful to include HL as a potential risk factor for falls in comprehensive falls risk assessments.
Strengths and Limitations
The strengths of this study include its strict adherence to a prespecified protocol and established guidelines for conducting systematic reviews and meta-analyses. Importantly, by only including studies that used covariate-adjusted estimates, we minimized confounding and achieved a lower degree of heterogeneity. This meta-analysis also examined both cross-sectional and longitudinal outcomes of falls and supported our findings through various subgroup and sensitivity analyses. Additionally, the absence of publication bias further enhances the reliability of our results.
There are some limitations of the present research. First, it is important to note that causal conclusions should not be assumed due to the observational nature of included studies, due to the possibility of residual confounding. Second, we were unable to examine how the severity of HL affected the prevalence and incidence of falls due to insufficient literature. Third, most studies relied on self-reported falls for practical purposes, which may be susceptible to recall bias. Fourth, self-reported measures of hearing and falls may exhibit greater variability compared with validated testing methods. This increased heterogeneity could influence the relationship between HL and falls. Future studies should strive to use validated methods for assessing hearing and identifying falls, which may aid in developing targeted strategies to reduce fall risk among individuals with HL. Nonetheless, our findings remained significant across subgroups using both self-reported and validated measurements of hearing. Fifth, due to insufficient reporting, we were unable to perform a subgroup analysis to investigate whether the association between HL and falls remained significant in adults of different age categories. Given that age was identified as a significant effect size modifier that accentuated the relationship between HL and falls, future studies may consider analyzing how the overall effect size differs between younger and older adults through subgroup stratification.
Conclusions
This systematic review and meta-analysis encompassing 27 studies and 5 071 935 participants suggests that HL may be associated with increased cross-sectional odds and longitudinal risk of falls. Given the significant public health ramifications of falls, physicians should be cognizant of the detrimental consequences of falls and recognize the importance of addressing hearing health as a potential mitigating strategy in fall prevention. The early identification of HL may aid in developing preventive strategies to reduce the incidence of falls.
eFigure 1. Leave-1-Out Analysis Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss
eFigure 2. Cumulative Analysis Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss
eFigure 3. Contour-Enhanced Funnel Plot Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss, With Missing Studies Imputed Via the Trim-And-Fill Method
eFigure 4. Linear Plot Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss
eFigure 5. Forest Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Stratified by Duration for Reporting Falls
eFigure 6. Forest Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Stratified by Continent
eFigure 7. Forest Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Only Among Individuals in the Community
eFigure 8. Leave-1-Out Analysis Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss
eFigure 9. Cumulative Analysis Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss
eFigure 10. Contour-Enhanced Funnel Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, With Missing Studies Imputed Via the Trim-And-Fill Method
eFigure 11. Linear Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss
eTable 1. Search Strategy
eTable 2. Summary of Patient Characteristics
eTable 3. Risk of Bias Assessment of Included Cross-Sectional Studies Using the Newcastle-Ottawa Scale (NOS)
eTable 4. Risk of Bias Assessment of Included Cohort Studies Using the Newcastle-Ottawa Scale (NOS)
eTable 5. Risk of Bias Assessment of Included Case-Control Studies Using the Newcastle-Ottawa Scale (NOS)
eTable 6. Evaluation of Quality of Pooled Evidence Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Framework
eTable 7. Results of the Meta-Regression Analyses
eTable 8. Results of the Publication Bias Analyses
Data Sharing Statement
References
- 1.James SL, Lucchesi LR, Bisignano C, et al. The global burden of falls: global, regional and national estimates of morbidity and mortality from the Global Burden of Disease Study 2017. Inj Prev. 2020;26(suppl 1):i3-i11. doi: 10.1136/injuryprev-2019-043286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2019 Ageing Collaborators . Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the Global Burden of Disease 2019 Study. BMJ. 2022;376:e068208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Step safely: strategies for preventing and managing falls across the life-course. Accessed September 15, 2024. https://iris.who.int/bitstream/handle/10665/340962/9789240021914-eng.pdf
- 5.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21(5):658-668. doi: 10.1097/EDE.0b013e3181e89905 [DOI] [PubMed] [Google Scholar]
- 6.GBD 2019 Hearing Loss Collaborators . Hearing loss prevalence and years lived with disability, 1990-2019: findings from the Global Burden of Disease Study 2019. Lancet. 2021;397(10278):996-1009. doi: 10.1016/S0140-6736(21)00516-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo BSY, Song HJJMD, Toh EMS, et al. Association of hearing aids and cochlear implants with cognitive decline and dementia: a systematic review and meta-analysis. JAMA Neurol. 2023;80(2):134-141. doi: 10.1001/jamaneurol.2022.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FR, Pike JR, Albert MS, et al. ; ACHIEVE Collaborative Research Group . Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet. 2023;402(10404):786-797. doi: 10.1016/S0140-6736(23)01406-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369-371. doi: 10.1001/archinternmed.2011.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deal JA, Reed NS, Kravetz AD, et al. Incident hearing loss and comorbidity: a longitudinal administrative claims study. JAMA Otolaryngol Head Neck Surg. 2019;145(1):36-43. doi: 10.1001/jamaoto.2018.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugraha S, Sabarinah S, Susilowati IH, Rahardjo TB. Intrinsic and extrinsic risk factor for fall among community dwelling Indonesian elderly. Open Access Maced J Med Sci. 2022;10(B):619-624. doi: 10.3889/oamjms.2022.8626 [DOI] [Google Scholar]
- 12.Huang RJ, Pieper CF, Whitson HE, Garrison DB, Pavon JM, Riska KM. Evaluating the association between hearing loss and falls in adults with vestibular dysfunction or nonvestibular dizziness. Ear Hear. 2022;43(3):1003-1012. doi: 10.1097/AUD.0000000000001156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. 2015;13(3):196-207. doi: 10.1097/XEB.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94-96. doi: 10.1136/bmj.39057.406644.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montero-Odasso M, van der Velde N, Martin FC, et al. ; Task Force on Global Guidelines for Falls in Older Adults . World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022;51(9):afac205. doi: 10.1093/ageing/afac205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 23.Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90(8):1067-1076. doi: 10.1097/ACM.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870 [DOI] [PubMed] [Google Scholar]
- 26.Alex D, Khor HM, Chin AV, et al. Factors associated with falls among urban-dwellers aged 55 years and over in the Malaysian Elders Longitudinal Research (MELoR) Study. Front Public Health. 2020;8:506238. doi: 10.3389/fpubh.2020.506238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assantachai P, Praditsuwan R, Chatthanawaree W, Pisalsarakij D, Thamlikitkul V. Risk factors for falls in the Thai elderly in an urban community. J Med Assoc Thai. 2003;86(2):124-130. [PubMed] [Google Scholar]
- 28.Hong Z, Xu L, Zhou J, et al. The relationship between self-rated economic status and falls among the elderly in Shandong Province, China. Int J Environ Res Public Health. 2020;17(6):2150. doi: 10.3390/ijerph17062150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseini SR, Zohani Z, Kheyrkhah F, Bijani A, Zabihi A. Relationship between falling and chronic diseases in the elderly: a study derived from Amirkola Health and Ageing Project. Iran Red Crescent Med J. 2020;22(8). doi: 10.32592/ircmj.2020.22.8.53 [DOI] [Google Scholar]
- 30.Jindal HA, Duggal M, Jamir L, et al. Mental health and environmental factors associated with falls in the elderly in North India: a naturalistic community study. Asian J Psychiatr. 2019;39:17-21. doi: 10.1016/j.ajp.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 31.Pantong U, Trapero I, Jareaprapal U. Analysis and prevention of falls among community-dwelling older adults in southern Thailand. J Adv Nurs. 2024;80(5):2121-2136. doi: 10.1111/jan.15945 [DOI] [PubMed] [Google Scholar]
- 32.Sakurai R, Kawai H, Yanai S, et al. Gait and age-related hearing loss interactions on global cognition and falls. Laryngoscope. 2022;132(4):857-863. doi: 10.1002/lary.29898 [DOI] [PubMed] [Google Scholar]
- 33.Shen S, Xie Y, Zeng X, et al. Associations of intrinsic capacity, fall risk and frailty in old inpatients. Front Public Health. 2023;11:11778132. doi: 10.3389/fpubh.2023.1177812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taheri-Kharameh Z, Poorolajal J, Bashirian S, et al. Risk factors for falls in Iranian older adults: a case-control study. Int J Inj Contr Saf Promot. 2019;26(4):354-359. doi: 10.1080/17457300.2019.1615958 [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Liu N, Zhao X. Assessing the relationship between hearing impairment and falls in older adults. Geriatr Nurs. 2022;47:145-150. doi: 10.1016/j.gerinurse.2022.07.007 [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Peng LN, Otsuka R, et al. Comparative analysis of intrinsic capacity impairments, determinants, and clinical consequences in older community-dwellers in Japan and Taiwan: longitudinal studies showing shared traits and distinct presentations. J Nutr Health Aging. 2023;27(11):1038-1046. doi: 10.1007/s12603-023-2020-z [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Hu Y, Luo J, et al. Association between sensory loss and falls among middle-aged and older Chinese population: cross-sectional and longitudinal analyses. Front Med (Lausanne). 2022;8:810159. doi: 10.3389/fmed.2021.810159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assi S, Garcia Morales EE, Du EY, Martinez-Amezcua P, Reed NS. Association of single and dual sensory impairment with falls among Medicare beneficiaries. J Aging Health. 2024;36(5-6):390-399. doi: 10.1177/08982643231190983 [DOI] [PubMed] [Google Scholar]
- 39.Riska KM, Peskoe SB, Gordee A, Kuchibhatla M, Smith SL. Preliminary evidence on the impact of hearing aid use on falls risk in individuals with self-reported hearing loss. Am J Audiol. 2021;30(2):376-384. doi: 10.1044/2021_AJA-20-00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiase VL, Tang K, Vawdrey DK, et al. Impact of hearing loss on patient falls in the inpatient setting. Am J Prev Med. 2020;58(6):839-844. doi: 10.1016/j.amepre.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 41.Zhao YL, Alderden J, Lind B, Stibrany J. Risk factors for falls in homebound community-dwelling older adults. Public Health Nurs. 2019;36(6):772-778. doi: 10.1111/phn.12651 [DOI] [PubMed] [Google Scholar]
- 42.Ogliari G, Ryg J, Qureshi N, Andersen-Ranberg K, Scheel-Hincke LL, Masud T. Subjective vision and hearing impairment and falls among community-dwelling adults: a prospective study in the Survey of Health, Ageing and Retirement in Europe (SHARE). Eur Geriatr Med. 2021;12(5):1031-1043. doi: 10.1007/s41999-021-00505-4 [DOI] [PubMed] [Google Scholar]
- 43.Skalska A, Wizner B, Piotrowicz K, et al. The prevalence of falls and their relation to visual and hearing impairments among a nation-wide cohort of older Poles. Exp Gerontol. 2013;48(2):140-146. doi: 10.1016/j.exger.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 44.Stam M, Kostense PJ, Lemke U, et al. Comorbidity in adults with hearing difficulties: which chronic medical conditions are related to hearing impairment? Int J Audiol. 2014;53(6):392-401. doi: 10.3109/14992027.2013.879340 [DOI] [PubMed] [Google Scholar]
- 45.Gopinath B, McMahon CM, Burlutsky G, Mitchell P. Hearing and vision impairment and the 5-year incidence of falls in older adults. Age Ageing. 2016;45(3):409-414. doi: 10.1093/ageing/afw022 [DOI] [PubMed] [Google Scholar]
- 46.Kiely KM, Khalatbari-Soltani S, Blyth FM, et al. Mixed evidence of an association between self-rated hearing difficulties and falls: prospective analysis of two longitudinal studies. Gerontology. 2023;69(1):98-108. doi: 10.1159/000524311 [DOI] [PubMed] [Google Scholar]
- 47.Lopez D, McCaul KA, Hankey GJ, et al. Falls, injuries from falls, health related quality of life and mortality in older adults with vision and hearing impairment–is there a gender difference? Maturitas. 2011;69(4):359-364. doi: 10.1016/j.maturitas.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 48.Tonelli M, Wiebe N, Lunney M, et al. Associations between hearing loss and clinical outcomes: population-based cohort study. EClinicalMedicine. 2023;61:102068. doi: 10.1016/j.eclinm.2023.102068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nugraha S, Prasetyo S, Susilowati IH, Rahardjo TBW. Urban-rural dimension of falls and associated risk factors among community-dwelling older adults. J Aging Res. 2021;2021:8638170. doi: 10.1155/2021/8638170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lord SR, Sherrington C, Menz HB, eds. Falls in Older People: Risk Factors and Strategies for Prevention. Cambridge University Press; 2001. [Google Scholar]
- 51.Jiam NT, Li C, Agrawal Y. Hearing loss and falls: a systematic review and meta-analysis. Laryngoscope. 2016;126(11):2587-2596. doi: 10.1002/lary.25927 [DOI] [PubMed] [Google Scholar]
- 52.Hattle M, Burke DL, Trikalinos T, et al. Multivariate meta-analysis of multiple outcomes: characteristics and predictors of borrowing of strength from Cochrane reviews. Syst Rev. 2022;11(1):149. doi: 10.1186/s13643-022-01999-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1-14. doi: 10.1016/S0966-6362(01)00156-4 [DOI] [PubMed] [Google Scholar]
- 54.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097-1118. doi: 10.1152/jn.2002.88.3.1097 [DOI] [PubMed] [Google Scholar]
- 55.Tell GS, Lefkowitz DS, Diehr P, Elster AD. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. Arch Neurol. 1998;55(1):73-79. doi: 10.1001/archneur.55.1.73 [DOI] [PubMed] [Google Scholar]
- 56.Lin FR, Ferrucci L, An Y, et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84-92. doi: 10.1016/j.neuroimage.2013.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vitkovic J, Le C, Lee SL, Clark RA. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21(4):195-202. doi: 10.1159/000445100 [DOI] [PubMed] [Google Scholar]
- 58.Agmon M, Lavie L, Doumas M. The association between hearing loss, postural control, and mobility in older adults: a systematic review. J Am Acad Audiol. 2017;28(6):575-588. doi: 10.3766/jaaa.16044 [DOI] [PubMed] [Google Scholar]
- 59.Foster JI, Williams KL, Timmer BHB, Brauer SG. The association between hearing impairment and postural stability in older adults: a systematic review and meta-analysis. Trends Hear. 2022;26:23312165221144155. doi: 10.1177/23312165221144155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Simonsick EM, Ferrucci L, Lin FR. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38(1):25-29. doi: 10.1016/j.gaitpost.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakurai R, Suzuki H, Ogawa S, Takahashi M, Fujiwara Y. Hearing loss and increased gait variability among older adults. Gait Posture. 2021;87:54-58. doi: 10.1016/j.gaitpost.2021.04.007 [DOI] [PubMed] [Google Scholar]
- 62.Kowalewski V, Patterson R, Hartos J, Bugnariu N. Hearing loss contributes to balance difficulties in both younger and older adults. J Prev Med (Wilmington). 2018;3(2):12. doi: 10.21767/2572-5483.100033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Amezcua P, Kuo PL, Reed NS, et al. Association of hearing impairment with higher-level physical functioning and walking endurance: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2021;76(10):e290-e298. doi: 10.1093/gerona/glab144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandemer L, Parseihian G, Kronland-Martinet R, Bourdin C. The influence of horizontally rotating sound on standing balance. Exp Brain Res. 2014;232(12):3813-3820. doi: 10.1007/s00221-014-4066-y [DOI] [PubMed] [Google Scholar]
- 65.Deviterne D, Gauchard GC, Jamet M, Vançon G, Perrin PP. Added cognitive load through rotary auditory stimulation can improve the quality of postural control in the elderly. Brain Res Bull. 2005;64(6):487-492. doi: 10.1016/j.brainresbull.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 66.Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab. 2013;98(1):51-58. doi: 10.1210/jc.2012-2119 [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Hu X, Zhang Q, Zou R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing. 2016;45(6):761-767. doi: 10.1093/ageing/afw140 [DOI] [PubMed] [Google Scholar]
- 68.Akinpelu OV, Ibrahim F, Waissbluth S, Daniel SJ. Histopathologic changes in the cochlea associated with diabetes mellitus–a review. Otol Neurotol. 2014;35(5):764-774. doi: 10.1097/MAO.0000000000000293 [DOI] [PubMed] [Google Scholar]
- 69.Fukushima H, Cureoglu S, Schachern PA, Paparella MM, Harada T, Oktay MF. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132(9):934-938. doi: 10.1001/archotol.132.9.934 [DOI] [PubMed] [Google Scholar]
- 70.Kurata N, Schachern PA, Paparella MM, Cureoglu S. Histopathologic evaluation of vascular findings in the cochlea in patients with presbycusis. JAMA Otolaryngol Head Neck Surg. 2016;142(2):173-178. doi: 10.1001/jamaoto.2015.3163 [DOI] [PubMed] [Google Scholar]
- 71.Crews RT, Yalla SV, Fleischer AE, Wu SC. A growing troubling triad: diabetes, aging, and falls. J Aging Res. 2013;2013:342650. doi: 10.1155/2013/342650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl 2):ii37-ii41. doi: 10.1093/ageing/afl084 [DOI] [PubMed] [Google Scholar]
- 73.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing. 2012;41(3):299-308. doi: 10.1093/ageing/afs012 [DOI] [PubMed] [Google Scholar]
- 74.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2):115-126. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529-535. doi: 10.1038/nature08983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wipperman J. Dizziness and vertigo. Prim Care. 2014;41(1):115-131. doi: 10.1016/j.pop.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 77.Paplou V, Schubert NMA, Pyott SJ. Age-related changes in the cochlea and vestibule: shared patterns and processes. Front Neurosci. 2021;15:680856. doi: 10.3389/fnins.2021.680856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cianfrone G, Pentangelo D, Cianfrone F, et al. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. 2011;15(6):601-636. [PubMed] [Google Scholar]
- 79.Kiely KM, Gopinath B, Mitchell P, Browning CJ, Anstey KJ. Evaluating a dichotomized measure of self-reported hearing loss against gold standard audiometry: prevalence estimates and age bias in a pooled national data set. J Aging Health. 2012;24(3):439-458. doi: 10.1177/0898264311425088 [DOI] [PubMed] [Google Scholar]
- 80.Blake AJ, Morgan K, Bendall MJ, et al. Falls by elderly people at home: prevalence and associated factors. Age Ageing. 1988;17(6):365-372. doi: 10.1093/ageing/17.6.365 [DOI] [PubMed] [Google Scholar]
- 81.Salari N, Darvishi N, Ahmadipanah M, Shohaimi S, Mohammadi M. Global prevalence of falls in the older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. 2022;17(1):334. doi: 10.1186/s13018-022-03222-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721-733. doi: 10.1016/j.neubiorev.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337-344. doi: 10.1001/jama.287.3.337 [DOI] [PubMed] [Google Scholar]
- 84.Tay L, Tay EL, Mah SM, Latib A, Koh C, Ng YS. Association of intrinsic capacity with frailty, physical fitness and adverse health outcomes in community-dwelling older adults. J Frailty Aging. 2023;12(1):7-15. doi: 10.14283/jfa.2022.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? Int J Audiol. 2013;52(5):360-368. doi: 10.3109/14992027.2013.769066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi JS, Adams ME, Crimmins EM, Lin FR, Ailshire JA. Association between hearing aid use and mortality in adults with hearing loss in the USA: a mortality follow-up study of a cross-sectional cohort. Lancet Healthy Longev. 2024;5(1):e66-e75. doi: 10.1016/S2666-7568(23)00232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riska KM, Peskoe SB, Kuchibhatla M, et al. Impact of hearing aid use on falls and falls-related injury: results from the Health and Retirement Study. Ear Hear. 2022;43(2):487-494. doi: 10.1097/AUD.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campos L, Prochazka A, Anderson M, Kaizer A, Foster C, Hullar T. Consistent hearing aid use is associated with lower fall prevalence and risk in older adults with hearing loss. J Am Geriatr Soc. 2023;71(10):3163-3171. doi: 10.1111/jgs.18461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Denham MW, Arnold ML, Sanchez VA, et al. Design and methods of the early age-related hearing loss investigation randomized controlled trial. Otol Neurotol. 2024;45(5):594-601. doi: 10.1097/MAO.0000000000004093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ganz DA, Latham NK. Prevention of falls in community-dwelling older adults. N Engl J Med. 2020;382(8):734-743. doi: 10.1056/NEJMcp1903252 [DOI] [PubMed] [Google Scholar]
- 91.Guirguis-Blake JM, Perdue LA, Coppola EL, Bean SI. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2024;332(1):58-69. doi: 10.1001/jama.2024.4166 [DOI] [PubMed] [Google Scholar]
- 92.Phelan EA, Ritchey K. Fall prevention in community-dwelling older adults. Ann Intern Med. 2018;169(11):ITC81-ITC96. doi: 10.7326/AITC201812040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Leave-1-Out Analysis Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss
eFigure 2. Cumulative Analysis Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss
eFigure 3. Contour-Enhanced Funnel Plot Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss, With Missing Studies Imputed Via the Trim-And-Fill Method
eFigure 4. Linear Plot Showing the Longitudinal Association of Hearing Loss and Incident Falls Compared With Individuals Without Hearing Loss
eFigure 5. Forest Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Stratified by Duration for Reporting Falls
eFigure 6. Forest Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Stratified by Continent
eFigure 7. Forest Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, Only Among Individuals in the Community
eFigure 8. Leave-1-Out Analysis Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss
eFigure 9. Cumulative Analysis Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss
eFigure 10. Contour-Enhanced Funnel Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss, With Missing Studies Imputed Via the Trim-And-Fill Method
eFigure 11. Linear Plot Showing the Cross-Sectional Association of Hearing Loss and Falls Compared With Individuals Without Hearing Loss
eTable 1. Search Strategy
eTable 2. Summary of Patient Characteristics
eTable 3. Risk of Bias Assessment of Included Cross-Sectional Studies Using the Newcastle-Ottawa Scale (NOS)
eTable 4. Risk of Bias Assessment of Included Cohort Studies Using the Newcastle-Ottawa Scale (NOS)
eTable 5. Risk of Bias Assessment of Included Case-Control Studies Using the Newcastle-Ottawa Scale (NOS)
eTable 6. Evaluation of Quality of Pooled Evidence Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Framework
eTable 7. Results of the Meta-Regression Analyses
eTable 8. Results of the Publication Bias Analyses
Data Sharing Statement