Abstract
The cornea is a specialized, transparent, avascular, immune-privileged, and heavily innervated tissue that affords 2/3rd of refraction to the eye. Ocular injuries, infections, and genetic factors affect corneal function and cause vision impairment. Presently, a variety of laser/non-laser surgeries, immunosuppressants, and/or corneal transplants are predominantly used to revive sight in human patients. The development of novel, precision-guided, and tissue-targeted non-surgical therapies promoting corneal repair and regeneration based on mechanistic understanding is of paramount importance to reduce the impact of global blindness. Research over the past decade revealed that modulation of pathological signaling pathways and factors by a variety of therapeutic delivery methods effectively treats corneal disorders including corneal scar/haze, inflammation, and angiogenesis in various pre-clinical animal models and are primed for human translation. This review discusses recent advances in the areas of corneal repair, restoration, and regeneration. Herein, we provide an overview of evolving approaches and therapeutic modalities that have shown great promise in reviving corneal transparency and function through the use of small drug molecules, gene therapy, nanomedicine, stem cells, trophic factors, exosomes, stromal equivalents, bioengineered stromal scaffolds, tissue adhesives, and 3D bioprinting.
Keywords: Cornea, Stromal regeneration, Stromal remodeling, Corneal wound healing, Corneal gene therapy, Keratocytes, Emerging therapies
1. Introduction
The cornea is a convex, aspheric, transparent, avascular, immune-privileged, and densely innervated tissue. Corneal thickness increases from the center to periphery and decreases with age. The cornea transmits light and provides two-thirds of refractive power to the eye. Trauma, injury, and infection to the eye cause varying degrees of corneal defects and visual impairment depending upon the severity. Corneal defects and diseases are the third most common cause of vision impairment in people globally (Allan, 1999; Huang and Li, 2007; Whitcher et al., 2001). An estimated 4.2 million people worldwide experience visually significant corneal opacities. The prevalence of corneal blindness varies across the globe and can show racial and geographic differences as well. Corneal blindness poses a significant impact on the quality and economic productivity of life (World Report on Vision, World Health Organization, 2021). Disorders like corneal scarring, haze, dry eye, neovascularization, keratitis, keratoconus, corneal dystrophies, herpes infection, chronic cicatrizing conjunctivitis, Stevens-Johnson Syndrome, pemphigoid, iridocorneal endothelial syndrome, advanced grades of pterygium and fibrosis are common corneal conditions that affect normal vision. Additionally, toxic gases, combat blasts, flying objects, traumatic brain injury and polytrauma are other sources of corneal injuries and blindness particularly in military personnel and veterans besides civilians (Flanagan et al., 2020; Frick and Singman, 2019). Corneal injuries induce complex wound healing responses to protect and restore corneal structure and transparency (Catala et al., 2021; Kamil and Mohan, 2021). Mild abrasions on the cornea can heal without the need for extensive tissue regeneration. However, deeper injuries can cause corneal scarring and visual disturbances. The uniform alignment of collagen fibers and the relative deturgescence of the corneal stroma helps maintain the optical clarity of the cornea. Alteration in corneal structure results in disruption of the characteristically organized collagen and causes scarring and opacities (Anderson et al., 2004; Fullwood, 2004). Induced irregular astigmatism, scar density, and associated corneal thinning can also contribute to the decrease in visual outcomes (Menda et al., 2020).
Corneal injuries may lead to the damage of epithelium, epithelial basement membrane (EBM), endothelium, and Descemet’s basement membrane (DBM). The treatment of the corneal disorder/disease would depend on the actual diagnosis and can include treatment with topical and systemic medications, phototherapeutic keratectomy using excimer laser ablation, transplant surgery, and rarely even the use of keratoprosthesis. Corneal injuries including refractive laser eye surgeries lead to stromal wound healing to facilitate wound closure and maintain transparency. This process is driven by many cytokines, growth factors, signaling pathways, and keratocyte conversion to myofibroblast. After the corneal insult, epithelial-derived cytokines, chemokines and growth factors, such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF) enter through the defective and injured EBM and activate quiescent keratocytes in the stroma, which transdifferentiate into metabolically active opaque light-scattering corneal myofibroblasts (CMFs) to influence wound repair by depositing high levels of extracellular matrix (ECM) components, collagens, and alpha-smooth muscle actin (α-SMA) stress fibers. Once the cornea heals, CMFs must disappear from the stroma to maintain transparency. Nevertheless, severe corneal trauma/injury often leads to undue generation and persistence of CMFs and deposition of excessive and irregular ECM components rendering the loss of corneal transparency (Barrientez et al., 2019).
Corneal stromal repair, regeneration and restoration are important for reinstating corneal transparency and visual recovery after ocular insult. Stromal regeneration therapeutics have been used to improve vision in human beings. It encompasses various therapeutic options including stem cell therapy, biomaterials, growth factors, tissue engineering, and replacement of diseased or damaged corneal tissue (Lightner and Chan, 2021; Maharajan et al., 2021; Pellegrini et al., 2009). However, there have been many challenges and hurdles associated with such treatments. Several approaches are developed and currently under investigation for corneal stromal repair (Oie and Nishida, 2013). These include various eye drops containing small molecule drugs, stem cells, gene therapy, nanomedicine, trophic factors, exosomes, stromal equivalents, bioengineered stromal scaffolds, tissue adhesives, and 3D bioprinting. Here we provide a glimpse of recent trends in corneal stromal repair and regenerative therapeutics.
2. Cornea structure and functions
The ocular surface includes the cornea, conjunctiva, lacrimal glands, and eyelids. The cornea is an important refractive media of the eye, in coordination with the lens of the eye, and focuses images on the retina. The cornea consists of cellular and non-cellular components. The cellular components include epithelial cells, keratocytes, and endothelial cells, and non-cellular components include collagen and glucosaminoglycans. The corneal epithelial cells are derived from the epidermal ectoderm. The keratocytes and endothelial cells are derived from the neural crest. The human cornea has mainly three layers, epithelium, stroma, and endothelium (Fig. 1). Bowman’s layer separates the epithelium and anterior stroma and Descemet’s membrane separates the posterior part of the stroma and the endothelial layer. Each of these corneal layers performs specific functions. The corneal epithelium is exposed to the outer environment and protects the underlying structures of the eye by functioning as a barrier against microbes, chemicals, and water. It provides a smooth optical surface along with the tear film. The stroma makes up about 90% of the cornea and regulates corneal transparency and refraction. The cornea protects the eye from dust, infective microorganisms, UV rays, and other foreign substances and provides the outer barrier of the eye. The cornea is a dome-shaped or prolate outer layer. If the cornea shape changes, it can cause nearsightedness, farsightedness, or astigmatism. The affected cornea can be reshaped by surgical treatments such as laser-assisted in situ keratomileusis (LASIK). The cornea is 11–12 mm horizontally and 9–11 mm vertically (McNutt and Mohan, 2020).
Fig. 1.

Schematic diagram showing human corneal anatomy. The cornea is avascular and consists of the outer epithelium made up of superficial cells, wing cells, and basal cells. Epithelium forms 10% of the cornea and protects the eye. Below the epithelium are the Bowman’s layer and stroma, which forms 85–90% of the cornea and contains collagen fibrils, ECM components, keratocytes, and nerve fibers. Stroma plays an important role in corneal homeostasis, repair, and transparency maintenance. Bowman’s layer is an acellular layer with ECM. Posterior to the stroma are Descemet’s membrane and a single layer of endothelium. Descemet’s membrane provides a resting structure for the endothelial cells. The endothelium controls corneal hydration and helps maintain corneal transparency.
The corneal epithelial layer (50 μm) is the outermost layer directly exposed to the outside environmental, physical, chemical, and pathogenic insults constantly. Corneal epithelial layer cellular homeostasis is maintained by limbal stem cells (LSC). This layer consists of nonkeratinized (less keratin), stratified squamous (flat) epithelial cells. The epithelial layer is made up of three epithelial cell types such as superficial cells, wing, and basal cells. Superficial cells consist of the top 3–4 layers of squamous cells (uppermost apical cells). The microvilli on the superficial surface increase corneal surface area and increase oxygen and nutrients from tears diffuse into the cornea. The middle 1–3 layers of the wing (flattened polygonal shape) shaped cells derived from basal cells. Basal cells are single layer of cuboidal/columnar cells with organelles and mitotic activities. The corneal epithelial cells have a life span of 7–10 days, undergoing involution, apoptosis, and desquamation. Below basal epithelial cells, a highly specialized acellular EBM consisting of ECM matrices (collagen, heparan sulfate proteoglycans, laminins and nidogens mostly) is positioned. The EBM with cross-linking of fibers and proteins intermingled with pores separates the epithelium from the stroma, supports tissue organization, and impacts wound repair (Torricelli et al., 2013). Beneath EBM starts stroma which constitutes 90% of the cornea and is composed of about 80% water by weight with parallel arranged lamellae of collagen I, IV, V in mucopolysaccharide matrix, proteoglycans, lumican, keratocan, mimecan and decorin, keratocytes, Langerhans” cells, dendritic cells, pigmented melanocytes, transient monocytes/macrophages and histiocytes (Kamil and Mohan, 2021; McNutt and Mohan, 2020). Underneath the EBM, lies Bowman’s layer (or membrane) in the corneas of humans, chickens, zebra fish, guinea pigs and other animals but not in all species. For example, it is absent in rabbits, felines, swine, and equine corneas. Bowman’s layer is composed of the randomly-oriented collagen fibrils but its role in corneal function and physiology is still unclear (Wilson, 2020a).
Keratocytes are the primary cell type in the stroma. These neural crest-derived cells are essential for the development of stroma by regulating/controlling the deposition of collagen fibrils and organizing lamella during stromal development. Keratocytes form a link between the lamellae with small cell bodies to minimize light scattering and synthesize stromal ECM. They reabsorb and resynthesize collagens, glycosaminoglycans, and produce matrix metalloproteases (MMPs) required for stromal homeostasis and repair mechanism. MMPs are also implicated in stromal degradation and ECM remodeling during wound healing. MMPs degrade ECM components and make the space for the new ECM components and cells to occupy the wound healing site. The corneal stroma also contains adult stem cells in the limbal region. Stroma provides mechanical strength, transparency, and refraction power. The cornea is one of the highly innervated and most sensitive tissues in the human body. The sensory nerves in the stroma are from the ophthalmic branch of the trigeminal nerve. This sensory nerve travels centrally and anteriorly in a radial path to the central cornea and the anterior and mid-stromal regions. The sensory nerve fibers innervate the basal epithelial cell layer and extend to superficial epithelial layers. Descemet’s membrane (10 μm) is located below the stroma and consists of collagen type IV. This membrane is continuously secreted by the endothelial cells. Descemet’s membrane acts as a resting layer for the endothelial cells. The endothelial layer is made up of a single layer of endothelial cells (cuboidal) with abundant mitochondria. Corneal endothelial cells have limited proliferation and regeneration potential in humans. The endothelium regulates corneal clarity by removing water from the corneal stroma (He and Bazan, 2016; McNutt and Mohan, 2020).
Stromal transparency is important for normal vision as it allows light to pass through it. Loss of normal transparency leads to corneal opacities followed by corneal scarring. Corneal transparency is regulated by the specifically organized ultrastructure, unique extracellular components, orderly distributed cells, absence of blood vessels, and restricted immune activities. Corneal opacity is caused by several cytokines, chemokines and growth factors produced from the epithelial cells, stromal cells, bone marrow-derived cells, and neuronal cells. Myofibroblasts and abnormal ECM from these cells play a significant role in the severity and persistence of opacity after corneal injury (Kamil and Mohan, 2021). Our ongoing in vivo confocal microscopy of stroma in human subjects reveal activation, migration, proliferation, and differentiation of quiescent keratocytes, and appearance/presence of fibroblasts and myofibroblasts in patients with corneal haze and presence of only quiescent keratocytes in subjects with clear/normal cornea (Fig. 2). Subjects with corneal haze also displayed several activated keratocytes, many fibroblasts and myofibroblasts, and abnormal collagen deposition in the stroma (Fig. 2). This human corneal stromal imaging data reaffirms our postulate that haze development encompasses various stages of the wound healing process and persistence of myofibroblasts post wound repair. Furthermore, this stromal imaging data from human subjects support the notion that targeting these events is a reasonable approach for drug discovery studies.
Fig. 2.

Representative in vivo confocal microscopy images of the corneal stroma of human subjects show the presence of only healthy quiescent keratocytes in clear/normal cornea (A), and activation, migration, proliferation, and differentiation of quiescent keratocytes, presence of activated keratocytes, fibroblasts, and myofibroblasts in hazy/opaque cornea (B–F). Corneal keratocytes are critical for stromal repair and corneal transparency.
3. Corneal homeostasis and wound healing
Corneal transparency is preciously maintained by the specialized ultrastructural design and function of epithelium, stroma, and endothelium, selected extracellular components, preciously distributed corneal cells, lack of blood supply and associated scanty immune surveillance is crucial to maintaining homeostasis for maintaining and restoring normal vision after various corneal disorders and diseases. Corneal transparency is primarily dependent upon the corneal stroma, especially the specific organization of collagen fibrils in the stroma (McNutt and Mohan, 2020; Meek and Knupp, 2015). Corneal keratocytes significantly contribute to the maintenance of corneal transparency and corneal shape by secreting and degrading the ECM. Corneal keratocytes are critical for homeostasis and transparency by the production of collagens, proteoglycans and crystallins (Fig. 3). Corneal keratocytes are quiescent with limited proliferation in the healthy cornea. Conversely, keratocytes in the injured cornea get activated and play a vital role in corneal repair. Immediately after injury, superficial keratocytes beneath the injured epithelium undergo apoptosis and create a thin sheet of dead cells to prevent the entry of toxins into the stroma in the absence of an epithelial barrier. This is the first observable event in the wounded cornea followed by activation of keratocytes under the influence of various cytokines and growth factors. The activated keratocytes called fibroblasts, act as a sensor and sense the damage-associated molecular patterns (DAMPs) from the damaged cells, pathogen-associated molecular patterns (PAMPs), inflammatory mediators, including cytokines, chemokines, trauma, and infections. Corneal fibroblasts express various cytokines, chemokines and adhesion molecules that selectively recruit limited inflammatory cells based upon the type of injury. Corneal fibroblasts can identify type 1 and type 2 inflammation. The corneal epithelial barrier is less permeable than the skin and protects the cornea from external injury, infections, and inflammatory mediators. Keratocytes can perform phagocytosis in the stroma. The stromal keratocytes are in contact with neighboring keratocytes through the process and make a network structure for communication. Thus, the resting keratocytes are in contact with other keratocytes and regulate ECM and stromal integrity. ECM in turn also controls the function of keratocytes. Thus, keratocytes and ECM interact and control each other for corneal homeostasis (Yam et al., 2020). The type I collagen that surrounds the keratocytes regulate keratocyte morphology, proliferation, function, and communication. Proteoglycans in the ECM help in the maintenance of stromal hydration, collagen fibrillogenesis, and keratocyte growth. Stromal ECM components include fibril forming collagens (I, III, V), fibril associated collagens (XII and XIV), network forming collagens (IV, VI and VIII) and small leucine-rich proteoglycans (SLRP) expressed in the stroma such as decorin, biglycan, lumican, keratocan, and fibromodulin (Espana and Birk, 2020).
Fig. 3.
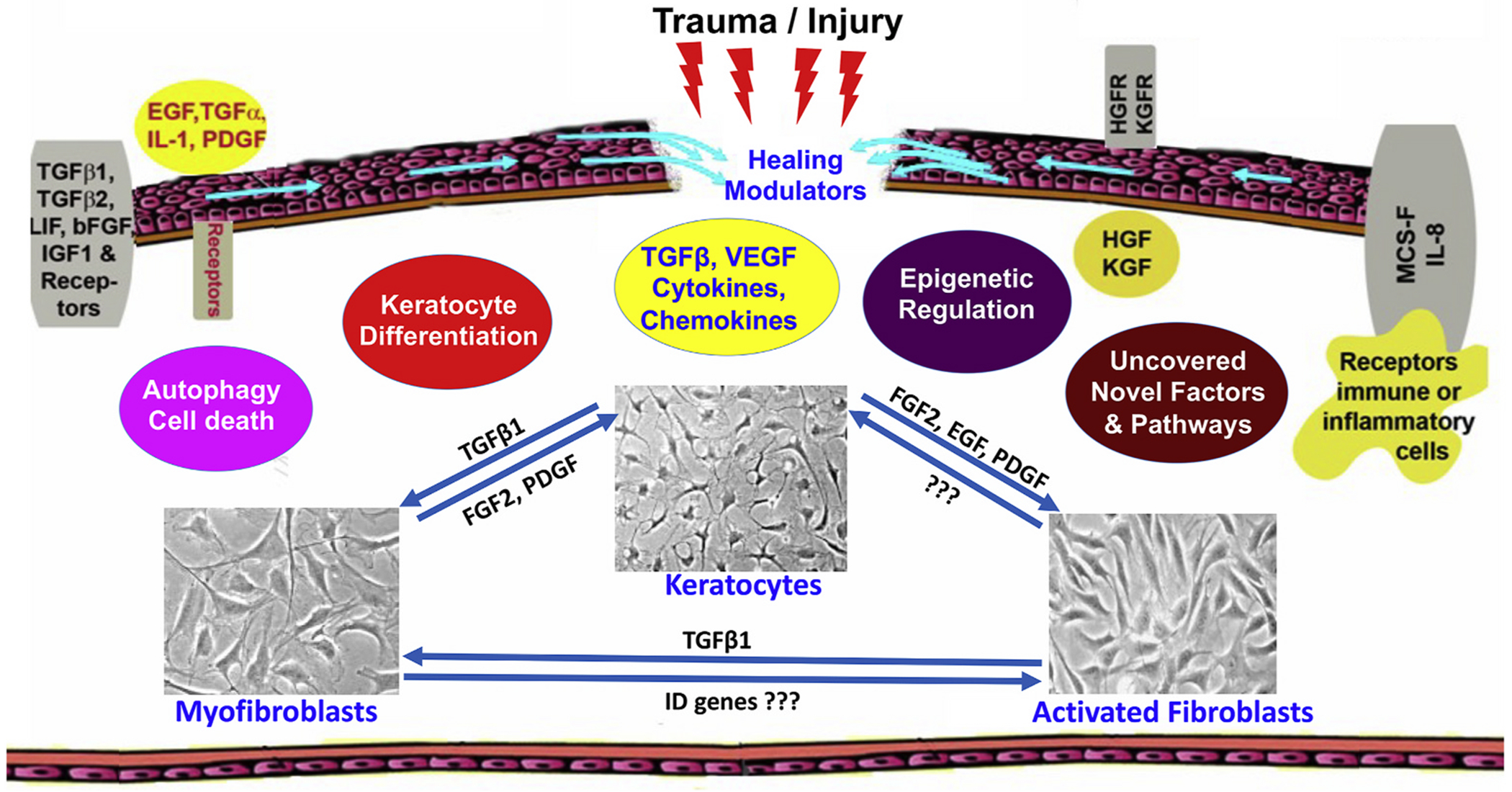
Schematic diagram showing events related to corneal repair, remodeling, and regeneration during wound healing after ocular trauma/injury. The cornea contains an array of cytokines, chemokines, and growth factors and their receptors which facilitate stromal repair and regeneration in an injured cornea. Keratocyte apoptosis, activation, proliferation, migration, and transdifferentiation to myofibroblast are controlled by many mechanisms to facilitate stromal repair, regeneration, and restoration. Myofibroblasts are a major cell type to perform these functions. Amount and timing of appearance/disappearance of myofibroblasts during/after wound repair dictate the pathological and physiological status of the cornea.
Keratocyte markers are keratocan, crystallins, and CD34. When the keratocytes are activated, these markers are reduced and become fibroblasts and then differentiate into myofibroblasts by acquiring actin-myosin bundles. Keratocytes synthesize and store crystallins in the cytoplasm and contribute to stromal transparency. Stroma also contains dendritic cells that perform phagocytic activities and present antigens to immune cells (Espana and Birk, 2020; Hamrah and Dana, 2007). Stem cells are found in the corneal stroma in the limbal stromal area. These stem cells can differentiate into keratocytes and these keratocytes can replicate. These stem cells are essential for the maintenance of epithelial cells. Corneal stroma also contains bone marrow-derived dendritic cells that are phagocytic, antigen-presenting, and other immune cells present in all the tissues (Hamrah and Dana, 2007). Dendritic cells play an essential role in ocular allergy, response to infection, and wound healing. Dendritic cells also interact with corneal nerves and accumulate around nerve fibers in the damaged cornea.
Corneal transparency after trauma/injury is affected by several cytokines, chemokines, growth factors, and conditions such as inflammatory response, fibrosis, neovascularization, and limbal disorders affect corneal transparency (Fig. 3). Though corneal immune privilege is essential to maintain corneal transparency, a limited immune response is essential for the wound healing process after an injury to restore normal vision (Mobaraki et al., 2019; Perez, 2017). The corneal function is regulated by apoptosis, necrosis, migration, proliferation, differentiation of corneal cells, and ECM modulation (Kamil and Mohan, 2021; Ljubimov and Saghizadeh, 2015). The microenvironment regulates immune responses by recruiting inflammatory cells to the cornea through local synthesis and release of chemokines. This process is dependent upon the extent of the injury. Mild corneal injury leads to regeneration of epithelium, keratocyte death, repair of epithelial basement membrane and DBM, apoptosis or conversion of myofibroblasts back to keratocytes or fibrocytes (Wilson, 2020c). If the corneal injury is severe, involving EBM and DBM, the profibrotic TGF-β, PDGF, cytokines, chemokines and growth factors enter the stroma and activate quiescent keratocytes to differentiate into contractile and opaque myofibroblasts (Ljubimov and Saghizadeh, 2015; Wilson, 2020b, c). IL-1 and TNF-α released from injured epithelial cells enter the stroma and induce activation or apoptosis of keratocytes as shown in Fig. 3. The cytokines, chemokines, metalloproteinases, keratinocyte growth factor (KGF) hepatocyte growth factor (HGF), and collagenases released from these keratocytes induce infiltration of monocytes, macrophages, lymphocytes, and fibrocytes into the site of injury, proliferation, and differentiation of epithelium. The cytokines, chemokines, and various growth factors released from epithelium, endothelium and keratocytes can take months and years to restore normal stromal function and corneal transparency after a severe injury. Also, any severe damage to the cornea damages the nerve fibers and reinnervation takes months to complete. Additionally, the myofibroblasts and fibrotic responses also inhibit the innervation process (Kamil and Mohan, 2021; Ljubimov and Saghizadeh, 2015; Mohan et al., 2012).
The corneal wound healing process is distinctive and different from other tissues. A healthy cornea is avascular and lacks blood and lymphatic vessels whereas most other organs including skin contain blood and lymphatic vessels to aid homeostasis and wound healing. Corneal wound repair is even distinct from skin though both cornea and skin tissues form an outer barrier and guard tissues/organs from external threats. Typically, corneal repair after mild to moderate injury does not involve the sprouting of blood vessels and capillaries. However, corneal repair post severe injury is associated with the ingrowth of neo blood/lymphatic vessels within the stroma from sclera/conjunctiva to augment the supply of healing factors such as vascular endothelial growth factor (VEGF), TGF-β, PDGF, IL-1, and fibroblast growth factor-2 (FGF-2). The VEGF and FGF-2 are known angiogenic factors and have been shown to foster neovascularization and irregular wound healing in the cornea after severe injury (Kamil and Mohan, 2021; Ljubimov and Saghizadeh, 2015).
Corneal stromal healing is modulated by both genetic and epigenetic factors. Unlike genetic alterations, epigenetic changes are reversible and do not change the deoxyribonucleic acid (DNA) sequence, but they can change how the body identifies a DNA sequence. We were the first to report modulation of TGF-β induced transdifferentiation of corneal fibroblasts and keratocytes to myofibroblasts and inhibition of corneal fibrosis in rabbits in vivo via epigenetics mechanism using an epigenetic modifier, Trichostatin A (TSA) (Fig. 4) (Sharma et al., 2009). Afterward, we showed the bench-to-bedside translational potential of this approach using an FDA-approved drug, suberoylanilide hydroxamic acid (SAHA), and underlying mechanism employing various preclinical in vitro and in vivo animal models (Fig. 5) (Anumanthan et al., 2018; Bosiack et al., 2012; Donnelly et al., 2014a; Gronkiewicz et al., 2016b; Sharma et al., 2009, 2015; Shetty et al., 2021; Tandon et al., 2012). Subsequently, we tested if SAHA can be an alternative for MMC by comparing the efficacy and long-term effects of these two drugs in an established rabbit in vivo model. Both SAHA and MMC efficaciously inhibited post-PRK corneal haze/fibrosis formation in rabbits in vivo. A most exciting finding was significantly high tolerability and reduced acute and long-term toxicity to the corneal endothelium by SAHA compared to MMC in rabbits (Fig. 6) (Anumanthan et al., 2017). Very recently, we compared acute and long-term effects of SAHA and MMC treatment on the human donor cornea, cultured limbal epithelial cells, corneal rims and lenticules collected from human subjects (Fig. 7) (Shetty et al., 2021). The results of this study demonstrated that SAHA alone and in combination with MMC (SAHA + MMC) deterred any loss of differentiation potential of corneal lineage cells when compared to MMC alone; SAHA treatment alone to lenticules was sufficient to reduce TGF-β induced fibrosis, and MMC alone treatment caused both short- and long-term adverse effects on cells and the cellular properties. Together, these results indicated that SAHA alone could effectively stop the generation of corneal haze after PRK surgery in patients without adverse effects like excessive cell death or compromised corneal cell differentiation (Shetty et al., 2021).
Fig. 4.

Representative images showing involvement of epigenetic mechanism in corneal fibrosis inhibition. TSA, a well-known epigenetic modifier, significantly decreased SMA and fibronectin expression in vitro (A–C) and PRK-induced corneal haze in vivo in rabbit cornea (D–M). SMA (green), fibronectin (red), and DAPI (blue), adapted from (Sharma et al., 2009).
Fig. 5.

Representative images showing bench-to-bedside potential of SAHA, an FDA-approved epigenetic modifier. A single treatment of SAHA (25 μM) after PRK on rabbit eyes significantly prevented the development of PRK-induced corneal haze in vivo (A–F) adapted from (Tandon et al., 2012), and the underlying mechanism used by SAHA for anti-fibrotic response (G–J) adapted from (Gronkiewicz et al., 2016b). Arrows show α-SMA (green), and f-actin (red). Western blot results show MAPKs and MMPs.
Fig. 6.

Representative images profiling SAHA versus MMC efficacy (A–F) and long-term safety (G–R) in vivo in rabbits after 1-month and 4 months post-PRK. Myofibroblasts (arrows), α-SMA (green); TUNEL positive cells = red (arrows), adapted from (Anumanthan et al., 2017).
Fig. 7.

Representative data displaying efficacy and safety of SAHA, MMC, or SAHA + MMC treatment on multidrug resistance proteins and limbal stem/progenitor cells derived from donor corneas, corneoscleral rims, and lenticules collected from human subjects. Representative FACS plots and quantification of ABCG2 (multidrug resistance protein) expression after SAHA, MMC, or SAHA + MMC treatment on cultured limbal epithelial cells differentiated to corneal lineage (A–G). Western blotting and quantification of the expression of the CK3/CK12, ΔNP63, COLL4, αSMA, BCl2 and GAPDH proteins after SAHA, MMC, or SAHA + MMC on limbal cornea epithelial cells isolated from corneoscleral rims of human subjects (H–I), adapted from (Shetty et al., 2021).
The focus of the current research remained on treating stromal opacity and corneal fibrosis after it develops or pre-treating the corneas empirically to reduce chances of haze development post-surgery. The corneal field still lacks the means of precisely predicting/identifying which human subjects might develop haze in the context of refractive laser surgeries or other corneal surgeries even though this knowledge is needed for patient-guided precision treatments. Recently, we discovered molecular factors that predispose patients to develop post-PRK haze. In this unique experiment, corneal epithelium from patients was collected at the time of surgery and grouped into those who developed post-surgical haze at 12 months compared to those that healed without any complications (Fig. 8) (Kumar et al., 2019). Transcriptomic and network analyses from these epithelium samples revealed several pathways and genes that were altered in those subjects that later developed haze. Among them, a novel gene PREX-1 was found to regulate fibrotic pathways, suggesting its use for precision medicine in the future (Fig. 8) (Kumar et al., 2019).
Fig. 8.

Representative clinical Slit-lamp images of the clear cornea 12 months post-PRK (A), grade 2 subepithelial corneal haze 12 months post-PRK (B), densitometry mapping of corneal haze by Oculus Pentacam (C–D) in human subjects, and microarray analysis of the pooled mRNA samples from haze predisposed and control groups (E–F). In this, corneal epithelium from patients was collected at the time of surgery and grouped into those who developed post-surgical haze at 12 months (A) compared to those that healed without any complications (B). Transcriptomic and ontological analyses found 1100 genes upregulated and 1780 genes downregulated in the haze predisposed group with changes in pathways regulating inflammation, oxidative stress, nerve functions, extra cellular matrix remodeling, and Wnt signaling. Factors like PREX1, SOX17, GABRA1, WNT3A, and PXDN showing significantly altered expression in haze predisposed subjects than with those of active haze subjects provoked us to conclude their pro-fibrotic role in corneal stromal wound healing and haze development, adapted from (Kumar et al., 2019).
4. Corneal stromal repair and regeneration mechanisms
4.1. Contribution of cellular machinery
Keratocytes are quiescent and transparent cells that float between the collagen lamellae and play a central role in stromal repair and regeneration and corneal transparency maintenance. Activated keratocytes produce collagen and proteoglycans and form the ECM after stromal injury. The human stroma consists of mainly collagen types I, V, and VI. Type I is predominant, followed by type VI. Type III collagen appears in inflammatory and wound healing events. Keratocytes also perform phagocytic activities and remove foreign particles from the stroma. Enzymes such as elastase and lipopolysaccharide released after insult activate keratocytes to secrete stroma-degrading MMPs, cytokines and chemokines that chemoattract immune cells to the stroma. Keratocytes actively participate in collagen degradation (Nishida, 2010).
Stromal remodeling is a complex mechanism and regulated by many factors, pathways and cytokines including pro/anti-fibrotic mediators such as TGF-β1, TGF-β2, HGF, EGF, FGF, and PDGF (Fig. 3). HGF renders many functions including anti-fibrotic activities. The FGF2 is shown to transform myofibroblast phenotype into fibroblast phenotype in vitro (Maltseva et al., 2001). The myofibroblasts derived from different sources differ in protein expression and functions. Defective and insufficient regeneration of EBM and DBM after an injury can also cause the persistence of myofibroblasts. Myofibroblasts produce ECM, growth factors, cytokines, and chemokines, and cause tissue contraction that regulates stromal cells including other myofibroblasts. Myofibroblasts prevent corneal nerve regeneration and induce the additional release of TGF-β from ECM. TGF-β acts through TGF-βR, Smad, mitogen-activated protein kinase (MAPKs) extracellular-signal-regulated kinase (ERK)/p38, c-jun N-terminal kinase (JNK), phosphatidylinositol 3-kinase (PI3K-Akt), Janus tyrosine Kinase-signal transducer, and activator of transcription (JAK-STAT) pathways (Kamil and Mohan, 2021). Intersecting Smad fibrotic signaling pathway by suppressing profibrotic Smads and/or overexpressing antifibrotic Smads was found effective in reducing fibrotic response in established in vitro and in vivo corneal fibrosis models (Gupta et al., 2017; Marlo et al., 2018). Also, selective sequestering of TGF-β signaling was found to be an attractive approach to treating corneal fibrosis. The viability of this approach was shown via the gene transfer approach by delivering anti-TGF-β genes such as soluble TGF-β type II receptor (sTGFβRII), bone morphogenic protein 7 (BMP7), decorin, Id3 etc. into corneal fibroblasts and via pharmacological intervention with pirfenidone, ITF2357 etc. (Fig. 9) (Fink et al., 2015; Lim et al., 2016; Sharma et al., 2012). The anti-fibrotic effects of anti-TGF-β genes provoked us to draw a novel postulate that simultaneous suppression of profibrotic Smads (Smad-2, -3, or -4) and overexpression of antifibrotic Smads (Smad 7) would have increased anti-fibrotic response. Intriguingly, the results of the study did not support the hypothesis as both single and combined Smad targeting did not improve anti-fibrotic response in the equine corneal fibrosis in vitro model (Fig. 10) (Fink et al., 2015; Lim et al., 2016; Marlo et al., 2018; Sharma et al., 2012).
Fig. 9.

Representative data showing the promise of a strategy involving selective sequestering of TGF-β signaling in corneal fibrosis/haze treatment in vitro and in vivo. Significantly reduced keratocyte/fibroblast transdifferentiation to myofibroblast in vitro and in vivo (A–L) and fibroblast migration in vitro (M–R) was observed in these experiments, adapted from (Fink et al., 2015; Gupta et al., 2020b; Sharma et al., 2012; Tandon et al., 2013).
Fig. 10.

Representative data showing effects of single and combined targeting of profibrotic Smads (Smad-2, -3, or -4) and antifibrotic Smads (Smad7) on corneal fibroblast differentiation. Both, single and combined, Smad targeting suppressed corneal fibroblast differentiation but combined targeting of Smads did not improve anti-fibrotic response in equine corneal fibrosis in vitro model, adapted from (Marlo et al., 2018).
Rapid stromal nerve fiber regeneration requires blocking myofibroblast differentiation and TGF-β1 release (Jeon et al., 2018). Stromal regeneration via keratocyte collagen synthesis is a slow process. It takes a long time for regenerated collagens to organize in proper orientation to conserve corneal transparency and shape. However, a short, large quantity of collagen deposition from myofibroblasts is associated with corneal haze and permanent stromal scar (Lagali, 2020). Further, corneal nerve regeneration is also critical for stromal regeneration. Therefore, stromal repair and regeneration strategies for therapeutic intervention should target the maneuvering of multiple pathways and mechanisms.
Proteoglycans participate and regulate collagen fibrillogenesis and matrix assembly (Gupta et al., 2022; Mohan et al., 2011c). The shape of corneal stromal cells is influenced by ECM. If the compact collagen is present around the keratocytes, they are quiescent with very limited mitotic activity and low proliferation capacity. Following trauma, inflammatory cells infiltrate the cornea and induce an inflammatory response with the release of inflammatory mediators such as IL-1 and TNF-α. Injury involving epithelial damage leads to enhanced production and availability of growth factors and cytokines such as EGF, TGF-β, IGF, and PDGF in a local microenvironment. Keratocyte apoptosis just beneath epithelium is the first observable wound healing event in the stroma after epithelial injury. Studies reveal that this process is driven by the IL-1, TNF-α, NF-kB, and Fas-Fas ligand system (Mohan et al., 2000, 2003a; Mohan and Wilson, 1999). Authors postulated that keratocyte apoptosis beneath epithelium after injury creates a sheet of dead cells which acts as a barrier to limit the entry of pathogens/toxins in the stroma and other ocular tissues. Another study indicated that reduction in keratocyte density in anterior stroma is compensated by the migration of keratocytes from peripheral stroma (Mohan et al., 2003b). Multiple cytokines and growth factors are largely afforded by epithelium at the site of injury to facilitate wound repair by activating keratocytes. TGF-β and PDGF in association with HGF, KGF, EGF, and other cytokines facilitate and regulate the migration, proliferation, and differentiation of keratocytes to activated keratocytes and myofibroblasts. The light-scattering, contractile, and metabolically active newly formed myofibroblasts synthesize and secrete a provisional matrix consisting of fibronectin, proteoglycans, and hyaluronan lead wound healing processes. Myofibroblasts contain α-SMA stress fibers in association with ECM lead wound healing and closure. Excessive formation and persistence of myofibroblasts in stroma after trauma, injury, or surgery cause corneal scars, haze, and/or fibrosis by producing abnormal ECM. Decorin, a glycoprotein, plays an essential role in stromal homeostasis and repair by antagonizing TGF-β and regulating collagen fibrillogenesis and ECM remodeling. Dysregulation of decorin leads to delayed wound healing. We previously reported decorin overexpression in human corneal fibroblasts intercepts TGF-β-induced myofibroblast trans-differentiation in vitro and corneal haze/fibrosis as well as neovascularization in rabbit cornea in vivo indicating that stromal wound healing events can be targeted for stromal repair/regeneration and to develop novel therapies to restore corneal function (Fig. 11) (Mohan et al., 2010, 2011b, 2011c, 2011d).
Fig. 11.

Representative data exhibiting that decorin gene overexpression in human corneal fibroblasts intercepts TGF-β-induced myofibroblast formation in vitro (A–D), adapted from (Mohan et al., 2010), and corneal haze/fibrosis in rabbit cornea in vivo (E–L) adapted from (Mohan et al., 2011b). These studies suggested that the corneal wound healing process can be easily targeted for stromal repair/regeneration and used to develop novel therapeutics to restore corneal functions. Green = SMA positive cells. E-I = representative stereomicroscope images.
The central cornea lacks lymphatic and blood vessels which essentially make this tissue immune privileged. Following severe injury, invasion of new blood vessels in avascular cornea termed as “corneal neovascularization” occurs from pre-existing peri-corneal structures due to an imbalance of angiogenic and antiangiogenic factors. is a common feature. Corneal neovascularization can cause scarring, edema, lipid deposition, and inflammation resulting in visual impairment. It also increases the risk of graft rejection after keratoplasty. Ideally, stromal wounds should heal without corneal neovascularization (Kamil and Mohan, 2021). Unfortunately, severe stromal injury leads to the ingrowth of blood and lymph vessels to augment the wound healing and repair process. The new blood vessel can grow from endothelial cells in the corneal limbus and can also through the cells that come from bone marrow. Blood vessels grow through the actions of growth factors such as VEGF, TGF-β, PDGF, IL-1, IL-6, integrins, MMPs, and FGF2 in the stroma that are released from various cells including corneal epithelial cells, keratocytes, and inflammatory cells. Vascular endothelial cells produce proteolytic enzymes that degrade the vascular basement membrane and nearby corneal ECM and thus can migrate into the stroma. These vascular endothelial cells proliferate while moving to the site where they sprout into the new blood vessel lumen and their branches in response to proangiogenic stimuli. However, in chronic inflammation, the new blood vessel becomes a permanent blood vessel which can suppress the immune privilege status of the cornea and exacerbate inflammatory response and fibrosis/haze. Neovascularization in the cornea is regulated by endostatin, angiostatin, arrestin, restin, metalloproteinase 3, and other factors (Kamil and Mohan, 2021). In an experimental model, neovascularization under the influence of VEGF implanted in a stromal micropocket in rabbit eyes takes place within three days after the insult, peaks on the 7th day and remains till the 14th day but starts to regress thereafter (Fig. 12) (Mohan et al., 2011d). This model is ideal for studying wound healing mechanisms and parameters associated with corneal neovascularization and identifying novel interventional strategies and developing newer therapies. We studied the functional role of decorin, a small leucine-rich proteoglycan, in managing corneal neovascularization in vivo. Results of this study revealed that decorin modifies stromal ECM and inhibits corneal neovascularization by altering the expression of pro- (VEGF, MCP1, and angiopoietin) and anti-angiogenic pigment epithelium-derived factor (PEDF) (Fig. 12) (Mohan et al., 2011d). Recently, Beclin-1, an autophagy gene, has been linked to corneal neovascularization regulation as Beclin-1 shRNA (short hairpin ribonucleic acid) blocked VEGF and neovascularization process (Zhu and Du, 2018). Substance P (SP) released from stimulated nerve fibers promotes the wound healing process and corneal neovascularization.
Fig. 12.

Representative images showing induction of corneal neovascularization by VEGF, and its inhibition by targeted decorin gene transfer into stroma in rabbits in vivo. A controlled time-dependent in-growth of blood vessels and neovascularization increase density in the avascular cornea was observed after VEGF-pellet implanted in the stroma (A, C, E) and rabbit corneas administration of decorin gene in stroma significantly reduced corneal neovascularization (A, C, E) by modifying stromal ECM. The decorin-delivered corneas in H & E staining showed vividly recovered corneal histology (G, H), reduced expression of CD31, an angiogenic marker, protein and mRNA (I, J), and rerecovered balance in pro-and anti-angiogenic genes (K), adapted from (Mohan et al., 2011d).
One of our recent studies performed with decorin knockout mice verified our hypothesis that decorin in the cornea regulates the balance of pro-angiogenic factors (Endoglin, PDGF, Pecam and VEGF) and anti-angiogenic factors (Ang2, Timp1 and VEGFR2) (Balne et al., 2021). Clinical Slit-lamp eye evaluations showed significantly higher corneal neovascularization in dccorin−/− mice than the decorin+/+ mice after chemical injury. Hematoxylin and Eosin staining and immunofluorescence revealed significantly high expression of α-SMA and endoglin proteins in the corneas of decorin knockout mice than in the wild type supported clinical observation. Further, significantly increased mRNA levels of pro-angiogenic factors Endoglin, VEGF, Pecam, and VEGFR2 in the cornea of the decorin−/− mice than the decorin+/+ mice provided additional support to this notion (Balne et al., 2021).
Recently, for the first time, we have demonstrated the expression and functional role of the calmodulin/calcium-activated K+ channels 3.1 (KCa3.1) in corneal wound healing using KCa3.1 deficient mice or primary human corneal fibroblasts were grown from donor corneas. (Anumanthan et al., 2018). The KCa3.1 deficient mice showed significantly reduced corneal fibrosis and expression of pro-fibrotic genes such as collagen I and α-SMA in vivo (Fig. 13) (Anumanthan et al., 2018). Additionally, we tested if blocking of KCa3.1 with triarylmethane-34 (TRAM-34, a specific inhibitor of KCa3.1) can be used to regulate stromal wound healing and inhibit fibrosis formation using an established in vitro model of human corneal fibrosis. The results of in vitro suggest that KCa3.1 regulates corneal wound healing and the blockade of KCa3.1 by TRAM-34 offers therapeutic strategies for corneal fibrosis as significant inhibition of TGF-β-mediated pro-fibrotic collagen I and α-SMA messenger ribonucleic acid (mRNA) and protein was observed (Fig. 14) (Anumanthan et al., 2018). This study offered the development of newer promising strategies to treat various corneal fibrotic disorders.
Fig. 13.

Representative images detecting KCa3.1 gene expression in human corneal epithelial, fibroblast, and endothelial cells by RT-PCR (A) and donor human cornea by immunofluorescence (B). KCa3.1 deficient mice showing distinctly reduced corneal haze post alkali insult compared to wild type in a time-dependent manner (C) indicated expression and functional role of KCa3.1 in corneal wound healing, adapted from (Anumanthan et al., 2018).
Fig. 14.

Representative phase-contrast microscopic data showing therapeutic promise of KCa3.1 controlling corneal fibrosis by pharmacological agent, TRAM-34 (a selective inhibitor of KCa3.1). Human corneal fibroblasts (HCF) grown in±of TRAM-34 and TGF-β1 and the effects of TRAM-34 were evaluated on fibroblast migration (A–F) and differentiation to myofibroblast (G–K). Treatment of TRAM-34 to HCFs demonstrated reduced fibroblast migration (A–F) and α-SMA (a fibrotic marker) levels in immunostaining (green; G-I) and western blotting (J, K), adapted from (Anumanthan et al., 2018).
4.2. Contribution of non-cellular machinery
The non-cellular machinery of the corneal stroma is comprised of characteristically arranged collagen fibrils (Fig. 15). It plays an important role in upholding corneal clarity, optical property, size, shape, biomechanics, and homogeneity of the refractive index (Copeland and Natalie, 2013; Meek, 2009; Meek and Knupp, 2015). The stroma contains bundles of collagen lamellae organized in a highly ordered manner. Stromal collagen lamellae are connective tissue made of parallel rows of fibrils, which mainly contain interweaved bundles of collagen type I. The predominant collagens in the stroma are type I, III, V, VI, and XIII. Collagen fibrils can have collagen type I heterodimerized with collagen type III or type V (Copeland and Natalie, 2013; Hassell and Birk, 2010; Zyablitskaya et al., 2020). Stromal lamellar fibrils are distinctively narrow with uniform diameter, span across the entire cornea, and are arranged at right angles with a high degree of lateral ordering. The precise assembly, alignment, and hexagonal pattern of fibrils are fundamental for normal corneal function including optical and biomechanical properties and irregularities in this pattern were observed in an injured opaque cornea (Fig. 15) (Michelacci, 2003; Zyablitskaya et al., 2020).
Fig. 15.

Representative transmission electron microscopy showing assembly, alignment, and packing of collagen fibrils in normal (A) and injured (B) mouse corneal stroma. A characteristic distribution, arrangement, and packing of collagen fibrils specific to corneal stroma were observed in normal cornea (A). On the other hand, the injured cornea demonstrated significantly altered assembly, distribution, and packing of collagen fibrils (B). Precise collagen fibrils organization is vital for maintaining corneal shape and optical property.
The interactions of collagens with stromal ECM organization play a critical role in maintaining intrinsic functional properties of the corneal stroma. However, research revealing a direct role of collagen fibrillogenesis during stromal wound healing and regeneration is limited. It is shown that collagen fibrillogenesis regulates collagen synthesis and metabolism that affects collagen fiber assembly and stromal structural integrity (Weis et al., 2005). The ECM has a prominent influence on cell behavior, shape, polarity, movement, metabolism, development, proliferation, and differentiation (Brown et al., 2002). Recent studies from our, and other, labs have presented some valuable data regarding the role of collagens on stromal transparency using wild-type and transgenic injured/uninjured mouse corneas and diabetic/non-diabetic porcine corneas (Sinha et al., 2021). Recent reports indicate that collagen XII deficiency in corneal stroma leads to significant stromal abnormalities including decreased interfibrillar space, disrupted lamellar organization, and increased corneal stiffness in their study performed with wild type and collagen XII deficient mice (Col12a1−/−) and alkali injury model of wound healing (Sun et al., 2020, 2021). Later, this group identified the role of collagen XIV in the early stages of stromal development and the modulation of stromal fibrillogenesis and wound healing in the adult cornea (Sun et al., 2021).
Literature reveals that insult to the cornea enhances interactions of collagens with the ECM components and increases the availability/activity of growth factors and cytokines including VEGF and TGF-β (Gronkiewicz et al., 2016b; Massague, 1998). These events lead to incompetent wound healing and foster fibrosis/scar formation in the cornea (Kamil and Mohan, 2021). How proteoglycans in the ECM interact and impact collagen architecture during wound healing stages remained poorly understood. A recent study indicates the spatial and temporal distribution of glycosaminoglycans and proteoglycans in mouse cornea after a chemical injury (Mutoji et al., 2021). Authors found significantly increased chondroitin sulfate and dermatan sulfate expression, decreased decorin expression, and unchanged heparan sulfate expression in the cornea after chemical injury and stated that injury to the cornea induces marked changes in the composition of the ECM. Interestingly, a recent study found that collagen stromal matrix perturbations are not limited only to chemical insult and can be induced by Type II diabetes as well. This was uncovered by our study in which Ossabaw mini pigs when fed a western diet exhibited compromised collagen fibrils arrangement along with the altered expression of genes associated with corneal wound healing (Fig. 16) (Sinha et al., 2021). Another recent study from our group performed using wild type and decorin knockout (dcn−/−) mice sought to understand the functional role of proteoglycan, decorin, in the regulation of collagen fibrils in response to injury at the ultrastructure levels with transmission electron microscopy and its relationship with corneal transparency during wound healing. Detection of significantly altered spatial packing of collagen fibrils and pro/anti-angiogenic/fibrotic factors in injured dcn−/− corneas compared to wild type corneas provided direct support that non-cellular collagenous machinery of the stroma plays an important role in stromal regeneration and corneal transparency restoration (Fig. 17) (Gupta et al., 2022).
Fig. 16.

Quantitative real-time PCR and transmission electron microscopy analyses comparing expression of collagen (A) and fibrosis (B) related genes and collagen fibrillogenesis (C, D) in normal and diabetic pig corneas. Age-matched normal and diabetic corneas of Ossabaw mini pig, a Type 2 diabetic animal model with a “thrifty genotype” were used in the investigation. None of the pig corneas showed any clinically relevant corneal haze. Nonetheless, detection of an altered expression of wound healing genes and mild change in stromal collagen fibrillogenesis divulges the vulnerability of the cornea to a diabetic condition, adapted from (Gupta et al., 2022).
Fig. 17.

Transmission electron microscopy showing the role of decorin in corneal stromal collagen fibrillogenesis in decorin deficient transgenic mice ± injury. The injured corneal stroma showed altered collagen fibrils arrangement and packing than the uninjured corneas. Violin graphs show inter-fibril distances (D), adapted from (Gupta et al., 2022).
4.3. Role of infiltrating immune cell machinery in the stroma
Being a mucosal barrier layer, the cornea has a heterogenous population of immune cells which likely utilize the capillaries and lymphatic vessels in the corneal limbus for trafficking. We have recently demonstrated that ocular surface immune cells such as neutrophils, natural killer (NK) cells, γδ-T cells, macrophages, etc. are altered in human patients with DED (Nair et al., 2021) and KC (D’Souza et al., 2021). However, most of the current knowledge regarding the role of immune cells in ocular surface healing comes from animal studies. Any wound in the cornea leads to the release of a variety of growth factors/cytokines (Li and Tseng, 1995) which orchestrate the timing and increase of tissue-resident cell migration and proliferation driving the healing response (Kamil and Mohan, 2021). These factors also activate and direct both resident and trafficking immune cells to the wound site in the cornea. Injuries or epithelial abrasions lead to the immediate influx of neutrophils to the site of injury (Sumioka et al., 2021). We and others have shown that mast cells are the effector cells in the innate and acquired immune response and are distributed ubiquitously in the body (Elieh Ali Komi et al., 2020; Kempuraj et al., 2016, 2019). Studies have also suggested that mast cells residing in the corneal limbus degranulate upon injury and add to the immediate inflammatory milieu of cytokines, chemokines and growth factors (Cho et al., 2020; Liu and Li, 2021; Mun et al., 2021), including CXCL2, which attracts neutrophils (Sahu et al., 2018). While mast cells have been implicated in the rejection of corneal transplants and mast cell inhibitors can reduce ocular surface inflammation, graft infiltration and conjunctivitis (Bielory et al., 2002; Elieh Ali Komi et al., 2018; Kempuraj et al., 2002; Mounsey and Gray, 2016), their inhibition prolongs corneal fungal infection and subsequent perforation and damage (Xie et al., 2018). Mast cell activation after injury induces corneal neovascularization (Cho et al., 2020). Dendritic cells (DCs), or Langerhans cells (LCs) in the basal epithelium can migrate along cytokine/chemokine gradients to the wound site (Gao et al., 2011; Niederkorn, 1995) as well as to the draining lymph nodes to activate the adaptive immune response. DCs are known to produce neurotrophic factors that could be important for the healing of corneal nerves (Choi et al., 2017) as well as epithelial homeostasis. The γδ-T cells are recruited to the corneal epithelium during the healing process (Li et al., 2007) while the macrophages migrate from the peripheral cornea to the injury sites over a few days (O’Brien et al., 1998). However, it has been reported that depletion of neutrophils and γδ-T cells impairs reepithelialization, which is dependent on IL-22 (Li et al., 2011). The reepithelialization process is also necessary for the reinnervation of the unmyelinated corneal sensory nerves and restoration of corneal homeostasis and reduction in ocular surface pain (Hegarty et al., 2018). Studies in murine models have shown the CCR2-macrophage subsets to be resident in the cornea whereas the CCR2+ macrophage subset is to be dependent on circulation (Liu et al., 2017). The CCR2+ subset carried the molecular signature of the pro-inflammatory M1 macrophages producing pro-inflammatory cytokines such as IL-1β, TNF-α, etc. The CCR2-subset expressed IL-10, Arg1, etc indicative of the M2 anti-inflammatory phenotype (Liu et al., 2017). As residents of the corneal limbi and conjunctiva, the NK cells contribute to the cytokine milieu by producing IL-17 and IFNγ and are required for the healing of corneal nerves and epithelial abrasions (Liu et al., 2012). However, NK cells have an inverse relationship with neutrophils, since excess neutrophils and inflammatory secretions prevent appropriate healing, indicating the delicate balance of immune cells to maintain homeostasis (Liu et al., 2012). NK cells and cytotoxic T-cells are usually associated with the elimination of infectious agents and their dysregulation may lead to chronic pathologies in the cornea. In scars due to chronic conditions, it is likely that there are imbalances between the inflammatory and regulatory cell populations. Elevated IL-17 in DED (Khamar et al., 2019) and autoimmune conditions such as Sjogren’s Syndrome and SJS are associated with the Th17 T cells that can suppress the regulatory T cells (T-regs) (Lee, 2018), subsequently leading to corneal pathologies (Coursey et al., 2017). T-regs can be enhanced by LCs (Price et al., 2015) which have been shown to be reduced in chronic corneal inflammation associated with a lack of neurotrophic factors and nerve damage. Interestingly, in keratitis, plasmacytoid DCs possibly protect the cornea by preserving the T-reg population (Yun et al., 2020). The waves of immune cell migration to the wound sites are important for the secretion of molecular factors required for the healing process (Fig. 18). Anti-inflammatory cells such as T-regs and M2 macrophages appear towards the end of the healing process to remove the inflammatory cells/secretions and phagocytose the apoptotic cells and other debris. Since imbalance of these cellular infiltration and activation processes are causative in cases of corneal opacities, their modulation may therefore be beneficial in treatment and prophylaxis.
Fig. 18.

Schematic depicting the role of the immune cell machinery in corneal wound healing. Upon injury, the immediate response begins with mast cell degranulation and immediate activation of neutrophils and the pro-inflammatory M1 macrophages. During the early phase, neutrophils, macrophages, and γδ-T cells migrate to the injury site and continue to reduce during the reparative phase. At this time, the wound is being actively healed via tissue remodeling. During this entire process, the dendritic cells are also activated and further interact with various immune cells during both the initial response and resolution phases. The anti-inflammatory M2 macrophages appear during the resolution phase and are important for adequate wound closure and completion of the healing process and return of corneal clarity. The imbalance within these cellular players as well as those not depicted here is often a driving factor for corneal scarring and fibrosis. Therefore, immunomodulation could play a critical role in optimal corneal wound healing.
5. Epidemiology and causes of corneal scarring
About 10 million patients are diagnosed with bilateral corneal blindness worldwide (Holland et al., 2021). The WHO reports that corneal blindness accounts for about 5.1% of blindness globally. Corneal scarring and neovascularization are responsible for about 4.9 million blindness and corneal ulceration and trauma for about 2 million blindness worldwide (Kumar et al., 2021). Corneal disorders account for vision loss in nearly 4% population of the United States and are the second leading cause of blindness in developing countries globally (Mohan et al., 2021b). Each year, corneal injury affects nearly 2.0 million people around the world (Burton, 2009; Whitcher et al., 2001). A variety of insults including ocular trauma, ocular infection, chemical injuries to the eye, ocular surgeries, and ocular acquired and inherited diseases lead to corneal blindness (Fig. 19). Stromal damage following trauma, injury, and/or infection to the eye leads to irreversible loss of corneal transparency and vision impairment. The non-surgical treatment options for repairing or restoring corneal transparency are limited and donor cornea transplant and keratoplasty remain a mainstay treatment to cure corneal blindness and restore vision in patients. Roughly 185,000 corneal transplantations are performed each year in 116 countries and 13 million people are waiting for corneal transplantation (Gain et al., 2016). Here, we review primary factors that have been implicated in mediating stromal injury, current clinical treatments, and emerging modalities/strategies to overcome stromal damage and lost function.
Fig. 19.

En face images of corneas from human subjects affected by fibrosis/haze of diverse etiologies. (A) Corneal Keratitis scar, (B) Scarring post corneal repair after trauma, (C) Diffuse corneal opacity with limbal stem cell deficiency post alkali burns, (D) Opacification of transplanted graft, (E) Corneal hydrops in advanced Keratoconus, (F) Corneal scarring in chronic sequelae of Stevens-Johnson syndrome, (G) Post PRK scar, and (H) Post keratoplasty suture scar.
5.1. Ocular trauma
Approximately half a million people worldwide have blindness secondary to ocular trauma (Wilson et al., 2012). Blunt and penetrating ocular trauma can result in corneal injury and scarring. The cornea may be damaged by chemical burns, ultraviolet radiation, extreme heat, electrical shock, scratches, blast waves and fragments due to blasts. Very often these occur at the workplace, mining injuries, agricultural accidents, road accidents and household accidents (Thylefors, 1992). Agricultural accidents can also be associated with contamination, resulting in additional corneal ulceration and visual loss (Thylefors, 1992). Active military service personnel, veterans, and civilians get corneal injuries through exposure to toxic gas such as sulfur mustard, hydrogen sulfide, and chlorine, and combat and terrorism-associated blasts, blast waves, infections, trauma/polytrauma, and TBI (Balne et al., 2020; Flanagan et al., 2020; Fuchs et al., 2021; Gupta et al., 2020a; Rasiah et al., 2021; Tripathi et al., 2020). Even after surgical repair of these corneal wounds, significant corneal scarring occurs along with the laceration and sutures. This is particularly important if the extent of the injury is large and involves the central cornea obscuring vision. These could require a corneal transplant to clear the central cornea. The scars also worsen the vision due to altered curvature and irregular astigmatism in these eyes. and need rehabilitation by contact lens (Shaughnessy et al., 2001) or excimer laser phototherapeutic keratectomy depending on the depth of the scar (Campos et al., 1993; Kollias et al., 2007). However, these measures can only be used for superficial scars, while deeper or full-thickness scars require some form of keratoplasty.
5.2. Corneal erosions or abrasions
Corneal erosion or abrasion is a superficial injury in which there is damage to the corneal epithelium and a break in its continuity. It can result in blurred vision with severe ocular pain. Corneal abrasion can occur from corneal scrapes/injuries, fingernail injuries, prolonged use of contact lenses, sport-related eye injuries, and blunt trauma to the eye. Corneal abrasions can also be recurrent in the eyes and are derived from stromal dystrophies, diabetes mellitus, ocular rosacea, nocturnal lagophthalmos, severe dry eye, cataract, and refractive surgeries (Ahmed et al., 2015; Miller et al., 2019). This entity is characterized by a derangement of anterior corneal ultrastructure which prevents adequate adherence of the epithelial cells to the underlying basal lamina and leads to corneal scarring. Corneal surgical procedures and preexisting ocular disease are also risk factors for perioperative corneal abrasion (Carniciu et al., 2017; Malafa et al., 2016). Chemical war agents such as sulfur mustard, chlorine, and hydrogen sulfide have been known to cause corneal abrasion and perforations (Panahi et al., 2017).
5.3. Corneal infections
Corneal infection is major ocular morbidity and blindness worldwide in developed and developing countries each year (Sharma et al., 2021; Whitcher and Srinivasan, 1997). Infections can be bacterial, fungal, viral, or acanthamoeba-related. Depending on the severity of the infection a varying degree of corneal scarring can persist even with the complete healing of the corneal ulcer (Hassan et al., 2017; Menda et al., 2020). Visual recovery from these corneal conditions includes the use of scleral contact lenses, phototherapeutic keratectomy, and PRK depending on the severity. The scar can also be associated with vascularization (discussed in the following sections) which increases the risk of rejection post corneal transplantation (Di Zazzo et al., 2017).
5.4. Chemical injuries
Ocular chemical injuries are a common cause of corneal haze and defects in a large population specially in people working in industries, laboratories, pesticide factories, agriculture, and cleaning workers. In severe cases, a complete loss of corneal function can be observed due to significant loss of keratocyte and limbal stem cells which produce these cells. Additionally, an increased immune response from infiltrating inflammatory cells such as monocytes and macrophages, migration of conjunctival epithelium centripetally and formation of a fibrovascular pannus. The ocular surface sequelae result in more scarring, poor visual prognosis, and response to therapy making them more challenging to rehabilitate (Dua et al., 2020). Treatment is generally tailored to amniotic membrane grafting at the acute phase and limbal stem cell transplant during the chronic phase with additional ocular surface reconstructive procedures as required. In the case of bilateral injuries, allogeneic stem cell transplants are considered, but that has the additional challenge of systemic immunosuppression and its associated complications (Agarwal et al., 2020). Due to the extremely dry surface in many of these patients, corneal transplants often do not perform well and may be rejected leading to scarring. These patients may then require a keratoprosthesis surgery to provide visual rehabilitation (Awasthi et al., 2021; Vasquez-Perez et al., 2018).
5.5. Ocular surgeries
Nearly 30 million people enduring vision impairment due to refractive error and cataracts opt for surgical intervention globally. Laser refractive surgeries like LASIK, PRK, and small incision lenticule extraction (SMILE) are popular around the world to treat nearsightedness, farsightedness, or astigmatism (Kumar et al., 2019). Both, PRK and LASIK, are unique and have pros and cons. The PRK procedure has regained popularity over LASIK in the last few years due to its long-term safety and fewer chances of ectasia (Vestergaard, 2014). The PRK involves corneal reshaping by surface ablation on stroma with/without epithelial removal with an excimer laser while LASIK involves a flap creation with laser or microkeratome followed by ablation in the mid stromal region with laser. In SMILE, the surgeon removes <4 mm corneal tissue (called lenticule) and creates a small incision on the corneal surface using a femtosecond laser to reshape the cornea and treat myopia. One of the known common complications of these procedures is postoperative scarring causing suboptimal visual recovery. Abnormal wound healing response after refractive surgery has been identified as a major reason for corneal haze development. Undue proliferation, migration, and differentiation of stromal keratocytes myofibroblasts, deposition of collagens, and defective ECM remodeling have been found to be major contributing factors to scar formation (Mohan et al., 2003a). Additionally, several clinical risk factors for haze development are also identified. These include high refractive error, ablation depth, ablation zone, and ultraviolet light exposure (Kundu et al., 2020; Rajan et al., 2006). The appearance of corneal haze resulting in blur vision is also common, though less frequent, in patients after glaucoma and cataract surgeries.
5.6. Keratoconus
Keratoconus (KC) is a degenerative corneal disorder associated with progressive thinning and steeping/protrusion of the cornea resulting in irregular astigmatism and deterioration of vision. KC affects corneal stromal integrity usually in the 2nd decade of life mostly in people suffering from ocular allergy, inflammation, and certain ocular syndromes (Santodomingo-Rubido et al., 2022; Shetty et al., 2015, 2020). The other risk factors include genetic factors, vigorous rubbing of the eye and ocular disorders with allergic responses. Elevated inflammatory factors like TNF-α, IL-6, and matrix metalloproteinase 9 (MMP-9) and reduced Lysyl oxidase (LOX) and Collagen IVA1 levels in corneal epithelium, stroma, and tears have been demonstrated by us and others in KC patients (Nishtala et al., 2016; Pahuja et al., 2016). Studies have also demonstrated dysregulated hormones/gonadotrophins in the KC corneas which have unique roles in modulating corneal pathology (Karamichos et al., 2021; McKay et al., 2022; Sharif et al., 2018). Our recent study demonstrating an altered ocular surface immune cell profile in KC patients highlights the interplay between chronic, aberrant immune response, and corneal stromal organization (D’Souza et al., 2021).
5.7. Stevens-Johnson syndrome
Ocular surface symptoms including severe corneal scarring and blindness are associated with the Stevens-Johnson syndrome (SJS). The eyes are affected in 40–84% of cases and show many ocular surface sequelae (Shanbhag et al., 2020). SJS is a type IV hypersensitivity immune-driven reaction that manifests as an acute blistering condition of the skin and mucous membranes. The exact pathophysiology of SJS and corneal symptoms arising from it is unknown. It is most commonly secondary to an idiosyncratic reaction to systemic medications or sometimes following a viral or mycoplasma pneumonia infection (Jain et al., 2016). The incidence rate of this condition ranges from 0.4 to 12.7 cases per million in the world every year (Frey et al., 2017; Kohanim et al., 2016b). These chronic ocular changes can range from minimal ocular surface involvement and symptoms to severe corneal scarring and blindness in end-stage disease (Kohanim et al., 2016a). The management of the condition would depend on the severity of the disease and the extent of corneal, conjunctival and lid involvement. It involves treatment of the associated dry eye and keratopathy, surgical procedures like mucous membrane graft to reduce the lid-related keratopathy, amniotic membrane transplant in case of persistent epithelial defects in addition to management of surface inflammation. Scleral contact lenses (PROSE) have also been useful in managing these patients (Kohanim et al., 2016a). Due to the poor surface stability, inflammation and extreme xerosis, the outcomes of corneal transplant can be poor in these cases. Such situations may require keratoprosthesis for visual rehabilitation (Kohanim et al., 2016b).
5.8. Inherited diseases
Genetically inherited corneal diseases affect corneal development and cellular function and in one or more layers of the cornea. Corneal dystrophies may lead to ocular pain, vision impairment, loss of visual acuity, lacrimation, and rarely total blindness. The 22 known corneal dystrophies are classified as anterior/superficial corneal dystrophies, stromal corneal dystrophies, and posterior corneal dystrophies. Each dystrophy exhibits uniquely distinctive histopathological and clinical manifestations (Constantin, 2021; Lisch and Weiss, 2020). Stromal health, function, and transparency are influenced by all dystrophies at varying levels. The risk factors for corneal dystrophies include gender, age, and genetics. The epithelial-stromal and stromal dystrophies include lattice corneal dystrophy, granular corneal dystrophy types I and II, Reis-Bückler’s corneal dystrophy, Thiel-Behnke corneal dystrophy (honeycomb dystrophy), macular corneal dystrophy, Schnyder corneal dystrophy, congenital stromal corneal dystrophy, fleck corneal dystrophy, posterior amorphous corneal dystrophy, pre-Descemet corneal dystrophy, and central cloudy dystrophy of Francois. Among all, Fuchs endothelial corneal dystrophy is more common with a prevalence of 3.7–11% across ethnicities and is characterized by loss of endothelial cell density and morphology and formation of guttae in the central endothelium in the early stages and can result in stromal scarring in the later stages (Krachmer et al., 1978; Vedana et al., 2016).
5.9. Keratopathies
Keratopathy is the term used to describe a disease of the cornea. It is associated with various local or systemic contributing factors. Several systemic diseases such as endocrine disorders, infective viruses and bacteria, autoimmune and inflammatory disorders, and genetic disorders affect cornea (Shah et al., 2021). It can range from a minor involvement of the cornea to a more severe sight-threatening condition. Neurotrophic keratopathy (NK) is a rare condition that develops from the defective trigeminal innervation to the cornea. NK is characterized by the loss of corneal sensation, impaired healing, persistent epithelial defects, and in severe cases corneal ulcer melting, perforation, and scarring. This condition is usually difficult to treat and is managed by supportive therapies. However, recently, surgical techniques such as nerve grafts have emerged (Liu et al., 2021). Diabetic keratopathy or diabetic corneal epitheliopathy is the most frequent and significant clinical disorder affecting human cornea in patients with systemic diabetic mellitus (Priyadarsini et al., 2020; Yu et al., 2022). These patients show the presence of corneal epithelial erosion, superficial punctate keratopathy, suppressed epithelial cell regeneration, decreased corneal sensitivity, reduced visual acuity and permanent vision loss (Barrientez et al., 2019; McKay et al., 2019, 2022; Priyadarsini et al., 2020; Yu et al., 2022). Diabetic keratopathies show abnormal wound healing and persistent corneal epithelial defects, unresponsiveness to treatment and specific pathogenesis is not clearly known. Various keratopathies including aphakic bullous keratopathy show the abnormal distribution of extracellular matrix in human corneas (Ljubimov et al., 1996).
5.10. Corneal melting
Corneal melting is a devastating complication of the end-stage corneal disease that can result in corneal perforation. It is triggered by excess production of tissue degradative proteases like MMPs. In this condition, the corneal epithelium is lost, and then the stroma becomes progressively thin and eventually leads to corneal perforation (Rigas et al., 2020). Corneal melting can be associated with systemic diseases, such as rheumatoid arthritis or lupus. Corneal melting is also common from prolonged/extreme exposure to chemicals, severe NK, somber fungal and viral keratitis. Another important cause of corneal melt is the indiscriminate use of topical nonsteroidal anti-inflammatory drugs (NSAIDs) (Rigas et al., 2020). Other causes are microbial keratitis, ocular surface disease, autoimmune disorders, and trauma (Deshmukh et al., 2020). Recently, a case of corneal melting was reported one week after cataract surgery in a patient who had undergone eyelid radiation and rheumatoid arthritis (Dervenis et al., 2021). Another recent case indicates corneal melting in a dry eye disease patient (Pchejetski et al., 2021).
6. Current treatments for clinical management of corneal scars
Treatment of corneal scarring and stromal abnormalities in patients depends on various factors including the severity, grade, depth of stromal injury, and underlying etiology. Ocular trauma, keratitis, chemical exposures/injuries, ocular surgeries, and keratopathies typically cause stromal wounds, inflammation, epithelial damage, corneal haze, and blurred vision. These conditions can be managed clinically using topical pharmacologic agents such as NSAIDs, steroids, doxycycline, MMC, tear supplements, biological preparations, immunomodulators, repurposed drugs, etc. (Fig. 20). Topical steroids and intraoperative MMC have been regularly used to prevent the incidence of postoperative haze following PRK haze (Arranz-Marquez et al., 2019; Jester et al., 1997). Treatment of postoperative PRK haze and scarring can be challenging to treat and can entail slow tapering of topical steroids and immunomodulatory agents like cyclosporine 0.05% and tacrolimus 0.03%. Repeat excimer ablation with topical or intraoperative MMC has been used to manage nonresponsive cases (Murueta-Goyena and Canadas, 2018). Anterior lamellar keratoplasty has also been described to treat recalcitrant stromal scarring in these cases (Tan and Ang, 2004).
Fig. 20.

Current therapies and emerging novel treatment strategies for corneal stromal repair and regeneration.
In the acute phase of severe chemical injuries, AMG (amniotic membrane graft) is performed to promote corneal epithelialization and reduce further complications (Shanbhag and Basu, 2021; Sharma et al., 2018). In the case of advanced corneal ulcers with perforation, cyanoacrylate tissue adhesives may be applied to close such perforations (Jhanji et al., 2011). In advanced ulcers with large perforations, procedures such as multi-layer AMG, tenon’s or corneal patch grafts may be required to manage the pathology (Kate et al., 2021).
The cases with dense scarring involving the visual axis may require corneal transplantation. A full-thickness scar would require a full-thickness penetrating keratoplasty while localized scars can be managed by lamellar keratoplasty (Chamberlain, 2019; Jhanji et al., 2012). Deep anterior lamellar keratoplasty (DALK) is performed by replacing most of the stroma with donor tissue, leaving behind the healthy recipient endothelium (Luengo-Gimeno et al., 2011). The outcomes of penetrating keratoplasty have been the current standard with greater than 70% of patients having good visual improvement postoperatively (Gain et al., 2016). There is, however, deterioration of postoperative vision and graft health over a long-term follow-up of more than 10 years (Williams et al., 2011). The causes of poor treatment outcomes include irreversible graft rejection and edema, secondary glaucoma, and graft infection. Lamellar techniques can have less risk of rejection and better surgical and visual outcomes (Alio Del Barrio et al., 2021b; Hos et al., 2019). Other challenges of the surgical interventions include limited availability of viable transplantable quality corneal tissue, high cost, long-term follow-ups, the skill of surgeons, and rejection of donor tissues due to transmissible diseases like Hepatitis B and C (Fuest et al., 2016; Gain et al., 2016; Mobaraki et al., 2019). If the grade of corneal scar is reduced, then conservative options like contact lenses can be used to improve visual outcomes and avoid corneal transplantation. The treatment of corneal deep scars with neovascularization also requires corneal transplantation. The other therapeutic options for this condition are the use of anti-angiogenic factors, anti-inflammatory agents, steroids, anti-VEGF, surgical removal of vessels, and gene therapy (Cursiefen and Hos, 2021; Keating and Jacobs, 2011; Mohan et al., 2021b; Nicholas and Mysore, 2021; Su et al., 2021). Intrastromal anti-VEGF therapy is more effective than subconjunctival therapy for corneal neovascularization (Ucgul et al., 2021).
Corneal stromal abnormalities at an early keratoconus stage are treated with eyeglasses or contact lenses. However, as the KC disease progresses, additional methods such as corneal collagen crosslinking and intra-corneal ring segments are used which are surgical methods due to the lack of topical treatment methods. In the advanced stages, there is extreme ectasia with subepithelial and stromal scar formation which adds to the decrease in vision and difficulty in fitting contact lenses for visual rehabilitation (Barr et al., 2006; Rabinowitz, 1998). As the disease severity advances, there is a loss of keratocytes reported in KC alongside abnormal activation of keratocytes and inflammatory signaling. As KC advances it can result in acute corneal hydrops, a stage where there is a break in the Descemet membrane resulting in corneal edema by ingress of aqueous in the corneal stroma (Tuft et al., 1994; Vohra et al., 2021a). Over time, the break heals with scarring which can vary depending on the area involved and the chronicity of the problem. The acute management of hydrops involves an injection of air or gas into the anterior chamber with or without compression sutures as a tamponade (Basu et al., 2011). Visual rehabilitation in advanced KC with scarring requires specialty contact lenses like the scleral lenses which can be very expensive for the patient or a corneal transplant which can be lamellar or penetrating depending on the depth and extent of scarring (Maharana et al., 2013).
The therapeutic options for corneal melting include the inhibition of collagenolytic effect on the stroma and treatment of the underlying condition like infection if any. Medications like acetylcysteine, ascorbate, and tetracyclines can have a beneficial effect in these cases. Corneal perforation management would depend on the size, shape, location, and cause of the lesion. Smaller lesions can be treated with tissue adhesives, Tenon’s patch grafting, or amniotic membrane transplantation. However, more extensive perforation may require keratoplasty (Deshmukh et al., 2020).
The management of ophthalmic manifestations including persistent epithelial defects, limbal stem cell deficiency, corneal neovascularization, dry eye, and corneal opacification from sulfur mustard exposure has been performed by amphoteric rinsing, dexamethasone, doxycycline and concurrent topical steroids with other therapeutic agents, such as nonsteroidal anti-inflammatory drugs, zinc-desferrioxamine (Fuchs et al., 2021; Tripathi et al., 2020). Recently, we developed a multimodal topical eye drop (TED) to treat acute mustard gas keratopathy (Tripathi et al., 2020).
7. Emerging novel therapeutic modalities for stromal repair and regeneration
A plethora of research is underway to develop newer effective and safe non-surgical methods to treat stromal defects and restore corneal function. Gene therapy is a fast-advancing modality and priming for clinical trials. The development of simple topical minimally invasive vector-delivery techniques allowing therapeutic gene delivery into desired cells/tissues is encouraging for the clinical translation of gene-based therapeutics in humans. However, many challenges including correct dosing of the therapeutic gene(s), the right timing of delivery, long-term safety, etc. are yet to be resolved. Here, we provide a brief overview of promising emerging therapies directed toward achieving stromal repair/regeneration and restoring vision (Table 1 and Fig. 20).
Table 1.
Current and emerging strategies for corneal wound healing, repair, and regeneration.
| Therapeutic strategy/administration | Therapeutic Genes/drugs | Corneal/stromal disorders | References |
|---|---|---|---|
| Penetrating keratoplasty | PK/DALK, keratoprosthesis | Corneal injury, blindness | Baradaran-Rafii et al. (2017) |
| Transplantation/stem cells | Limbal stem cells, adipose stem cells, MSCs, anti-inflammatory cytokines | Graft rejection, Corneal injury, Stromal regeneration | (Baradaran-Rafii et al., 2017; Basu et al., 2014; Mohan et al., 2021b; Soleimani and Naderan, 2020) |
| Tear supplements | Hyaluronan, artificial tears | Dry eye disease, epithelial erosions | (Di Iorio et al., 2019; Manzur Yarur et al., 2021; Soleimani and Naderan, 2020) |
| Biological preparations | Autologous serum, umbilical cord serum, PRP | Chemical burns | (Manzur Yarur et al., 2021; Soleimani and Naderan, 2020) |
| Antibody therapy | Anti-VEGF, anti-IL-6 | Wound healing | (Baradaran-Rafii et al., 2017; Sharma et al., 2018; Soleimani and Naderan, 2020) |
| Vitamins | Vitamin A, Vitamin C | Blindness, wound healing | (Aghaji et al., 2019; Kim et al., 2012) |
| Antibiotics and chemicals | Tetracyclines, citrate, cysteine, acetylcysteine, EDTA, penicillamine | Graft rejection | Di Iorio et al. (2019) |
| Repurposed drugs | Tranilast, SAHA, TSA, cyclosporine A, Doxycycline | Inhibit corneal fibrosis & excess wound healing | (Gronkiewicz et al., 2016a; Sharma et al., 2016) |
| Immunomodulators | Resolvin E1, Monocyte inhibitor PRM151, steroids, IL-1 receptor antagonist | Anti-inflammatory, anti-angiogenesis, decrease myofibroblast generation | (Jin et al., 2009; Santhiago et al., 2011; Sharma et al., 2021; Stapleton et al., 2008) |
| Antifibrotic agents, Stromal repair | anti-TGFβ, KCa3.1 | Inhibit corneal scarring/fibrosis | (Anumanthan et al., 2018; Di Iorio et al., 2019; Kamil and Mohan, 2021; Sharma et al., 2018; Tandon et al., 2010; Torricelli et al., 2016) |
| Gene Therapy | HGF + BMP7, Decorin, Decorin-PEI, VEGFR, Smad7, HLA-G, Endostatin, sVEGFrll, Ad. GusB, siRNA | Inhibition of fibrosis/scarring, angiogenesis/neovascularization. Restores transparency | Amador et al. (2021); Balne et al. (2021); Chaudhary et al. (2014); Di Iorio et al. (2019); Gupta et al. (2018); Gupta et al. (2017); Hirsch et al. (2017); Marlo et al. (2018); Mohan et al. (2021a); Mohan et al. (2021b); Mohan et al. (2013); Mohan et al. (2011a); Mohan et al., 2012; Rodier et al. (2019) |
| Growth factors | NGF, HGF | Dry eye disease, fibrosis, neurodegeneration, corneal nerve repair & increased sensitivity | (Gong et al., 2021; Kanu and Ciolino, 2021; Medeiros and Santhiago, 2020) |
| Corneal wound healing | Neprilysin (NEP) inhibition | Corneal injury | Genova et al. (2018) |
| Bioengineered Corneal stroma/stromal replacement | Stromal equivalents, Decellularized scaffolds, recellularized scaffolds, cell-free scaffolds, tissue adhesives/hydrogel, cell sheet, gelatin, synthetic polymers, fish scale, silk fibrin, collagen, 3D bioprinting | Stromal regeneration, ECM remodeling, collagen production, stromal transparency | (Alio Del Barrio et al., 2021a; Brunette et al., 2017; Lagali, 2020; Matthyssen et al., 2018; Mobaraki et al., 2019) |
| Autophagy modulation, Autophagic activator | Rapamycin | Wound healing and repair, inhibit fibrosis and angiogenesis, ocular health | (Martin et al., 2019; Wang et al., 2020) |
7.1. Gene therapy modalities
Gene therapy is an attractive approach for curing corneal disorders (Mohan et al., 2021b). Gene therapy entails the introduction of genetic material into corneal tissues to express a therapeutic protein to prevent, treat or cure inherited/acquired diseases and disorders. Restoration of normal vision by gene therapy depends upon many factors, including vector and gene delivery into targeted cells/tissues of the cornea (Mohan et al., 2012). The cornea is a perfect tissue for gene therapy due to its convenient accessibility and ease of monitoring. Viral and non-viral vectors including nanoparticles have been tested to deliver gene therapy for treating corneal scarring/fibrosis and neovascularization.
7.1.1. Gene therapy using viral vectors
Multiple recombinant viral vectors such as adenovirus (rAV), retrovirus (rRV), lentivirus (rLV), herpes simplex virus (rHSV), and adeno-associated virus (rAAV) were examined for introducing DNA into the corneal cells in vitro, in vivo and ex vivo conditions (Mohan et al., 2012). Our group has evaluated the potential of rLV and cellular tropisms and transduction efficiency of AAV2, AAV5, AAV2/5, AAV2/6, AAV2/8 and AAV2/9 for delivering gene therapy into corneal stromal cells and corneal endothelial cells derived from human, rabbit, rodent, and canine corneas employing in vitro, ex vivo, and in vivo models (Mohan et al., 2013, 2021b; Sharma et al., 2010a). Additionally, we have developed many minimally invasive vector-delivery techniques to introduce tissue-selective gene therapy in the cornea (Mohan et al., 2012). Also, we successfully optimized efficacious and safe tissue-selective controlled gene therapy methods to deliver therapeutic genes in a tissue/site targeted manner in the rabbit cornea in vivo testing various combinations of an rAAV vector and simple vector-delivery techniques (Mohan et al., 2011a). We found that the relative transduction efficiency of pseudotyped AAV2/6, AAV2/8 and AAV2/9 vectors in the order of AAV2/6 > AAV2/9 > AAV2/8 for primary human corneal fibroblasts in vitro but for the mouse cornea in vivo and donor human cornea ex vivo it was AAV2/9>AAV2/8>AAV2/6 (Sharma et al., 2010a, 2010b). Also, the efficiency of AAV2/5 was found highly efficient for introducing transgenes into human corneal fibroblasts in vitro and in ex vivo organ culture models, rodent, and rabbit cornea in vivo (Mohan et al., 2005, 2011b, 2011d). Subsequently, we evaluated the translational potential of various therapeutic genes by delivering them into stroma employing optimized tissue-targeted gene delivery methods which are discussed later in the section.
TGF-β signaling has been identified as a major mechanism for the development of corneal scarring in vivo. Thus, we have tested various genes that can impede TGF-β signaling to treat corneal scarring. We found a single topical administration of AAV2/5-Smad7 into rabbit stroma effectively inhibited PRK-induced corneal haze and corneal fibrosis in vivo without immune cell infiltration or visual toxicity (Gupta et al., 2017). Our earlier studies found significant inhibition of corneal fibrosis and neovascularization in rabbits by localized targeted delivery of decorin gene with rAAV2/5-decorin vector (Mohan et al., 2011b, 2011d). Our recent study investigated the 6-month toxicity profiling of decorin therapy for the eye using a rabbit model and concluded that topical AAV5-decorin gene therapy was found to be safe and nontoxic to the rabbit eye (Fig. 21) (Mohan et al., 2021a). Decorin is a suppressor of TGF-β signaling pathways. Myofibroblast and haze formation in the cornea is due to an increased release of TGF-β from the corneal epithelium following injury to the eye.
Fig. 21.

Representative data showing 6-month toxicity profiling of AAV5-decorin gene therapy to the eye in a rabbit model. Representative images of in vivo stereo microscopy (A, B), slit-lamp microscopy (C, D), H & E staining (E, F), and in vivo confocal microscopy in the corneal epithelium, stroma and endothelium (G–P), suggested that targeted AAV5-decorin gene therapy to of cornea is safe and tolerable at least up to 6-month in vivo in rabbits, adapted from (Mohan et al., 2021a).
These studies not only validated our postulate that multiple signaling pathways could be targeted during an active wound healing state but also offered to formulate novel innovative strategies to develop non-surgical regenerative medicine options to restore stromal damage and restore corneal clarity. AAV gene therapy using human leukocyte antigen G (HLA-G) genes has been shown to reduce corneal neovascularization and scarring (Hirsch et al., 2017). Corneal gene therapy has also been done in the canine model of the genetic disease Mucopolysaccharidosis type I (MPS I), a lysosomal storage disease characterized by corneal haze. Since the disease is caused due to mutations in alpha-l-iduronidase (IDUA), rAAV was used to deliver a healthy copy of IDUA which reduced corneal scarring (Miyadera et al., 2020). Gene therapy approaches have also been attempted for controlling corneal graft rejection by expressing immunomodulatory genes (Ritter et al., 2013), however, given the transient nature of the rejection process, further studies regarding temporal control of such approaches are required.
7.1.2. Gene therapy using non-viral vectors
Non-viral gene therapy for corneal disorders is also tested using physical techniques, chemicals, naked DNA, electrical/surgical methods, gene gun, hydrodynamics, electroporation, iontophoresis, ultrasonography, lasers, microinjection, and stromal hydration (Mohan et al., 2013). Microinjection of plasmid via a needle 0.2 μm or less in diameter successfully delivered genes in the cornea (Amador et al., 2021; Mohan et al., 2021b). Likewise, high-intensity electric impulses, ionized molecules, and creation of pores in the cell membrane delivered genes in corneal tissue (Mohan et al., 2021b). The non-viral gene transfer methods offered therapeutic benefits of higher safety, delivery of large therapeutic genes, and lower immunogenicity but had many limitations including low transfection efficacy, aggregation in blood and body fluids, colloidal instability, gene breakdown, and ocular toxicity (Amador et al., 2021; Mohan et al., 2021b).
7.1.3. Nano therapy
To develop non-viral gene therapy approaches, we tested various nanoparticles, plasmids, peptides, and lipids for delivering therapeutics in the cornea (Kanwar et al., 2012; Mohan et al., 2012, 2021b). Among various evaluated non-viral vectors, we identified and expanded the development of non-viral gene therapy for corneal stromal disorders with polyethyleneimine-conjugated gold nanoparticles (PEI2-GNPs), 2–12 nM in size. PEI2-GNPs vector demonstrated a remarkably high ability to deliver transgenes with low toxicity into corneal fibroblast and endothelial cells of the human cornea in vitro and ex vivo and rodent and rabbit cornea in vivo (Mohan et al., 2012, 2021b; Sharma et al., 2011). Interestingly, PEI2-GNP vector was also found highly efficient for delivering transgene into rat peritoneum in vivo without showing major toxicity (Chaudhary et al., 2014). This study was performed in collaboration with a group of nephrologists to assess the clinical potential of the decorin gene for treating peritoneal fibrosis using a standard rodent in vivo model.
Utilizing PEI2-GNP vector, our subsequent studies evaluated the therapeutic translational potential of various genes including BMP7, HGF, decorin, Smads, and others delivered single or in combination to treat corneal fibrosis and facilitate stromal repair/regeneration using a well-developed preclinical in vivo rabbit model and standard in vitro human, equine, and canine corneal disease models of corneal wound healing (Bosiack et al., 2012; Buss et al., 2010; Chaudhary et al., 2014; Donnelly et al., 2014b; Gupta et al., 2018; Mohan et al., 2011b, 2021b; Rodier et al., 2019; Sharma et al., 2011, 2012; Tandon et al., 2013). Targeting pathways influencing keratocyte proliferation and differentiation following corneal injury by multifunctional cytokines has been an innovative approach to modulating corneal stromal wound healing in vivo. Following this strategy, theBMP7 gene was introduced into an injured rabbit stroma in vivo via PEI2-GNPs; and it prevented undue wound healing and reduces corneal fibrosis in a rabbit in vivo disease model. The findings of this led to a novel hypothesis that targeting multiple factors and/or signaling pathways can be used to promote stromal regeneration and restore corneal transparency in vivo. We tested this concept by delivering two genes, BMP7 and HGF, with PEI2-GNPs in rabbit stroma in vivo. In this study, we evaluated the therapeutic potential of BMP7 and HGF combined gene therapy to treat pre-existing corneal fibrosis using a rabbit in vivo model. Corneal fibrosis was induced by alkali injury in rabbits, and 24 h after the scar formation, BMP7 and HGF genes were administered with PEI2-GNPs. We have detected significantly reduced mRNA levels of profibrotic genes, α-SMA, fibronectin, collagen I, collagen III, and collagen IV compared to the non-treated rabbit corneas (Fig. 22) (Gupta et al., 2018). A subsequent study evaluating the long-term safety of BMP7+HGF therapy found it safe and tolerable to the rabbit eye for up to the longest tested 7-month time (Fig. 23) (Gupta et al., 2021; Mohan et al., 2021a).
Fig. 22.

Representative data showing effects of BMP7+HGF nanomedicine in controlling corneal fibrosis in vivo in a rabbit model. The corneas with the delivery of BMP7+HGF nanomedicine showed significant abrogation of alkali-induced corneal fibrosis/haze and restoration of corneal transparency in live animals in slit-lamp examination (B, D, F) after 21 days compared to no therapy given corneas (A, C, E). Histological H & E staining and immunofluorescence for α-SMA (green) supported the anti-fibrotic effects of BMP7+HGF nanomedicine (J, K) as significantly improved corneal health and reduced α-SMA (green) were observed compared to untreated corneas (G, H). Double immunofluorescence analysis of α-SMA+ (green) and TUNEL + cells (red) showed a prospective underlying mechanism employed by BMP + HGF, adapted from (Gupta et al., 2018, 2021).
Fig. 23.
Representative data showing 7-month-long tolerability of BMP7+HGF nanomedicine in vivo in rabbit eyes. In vivo confocal images of rabbit corneal epithelium, stroma, and endothelial layers in naïve (A–E), naked vector delivered (F–J) and BMP-HGF (K–O) suggest that BMP7+HGF nanomedicine is safe and nontoxic to rabbit eyes. Line graphs showing time-dependent analyses of central corneal thickness (CCT) with pachymetry (P), tear flow with Schirmer Tear Test Strips (Q) and intraocular pressure (IOP) with tonometry (R) highlight the safety and tolerability of BMP + HGF nanomedicine in rabbit eyes up to 7 months, adapted from (Gupta et al., 2021).
7.1.4. Gene editing
CRISPER/Cas9 gene editing is an emerging novel gene therapy approach to treat acquired and genetic ocular disorders/diseases including corneal defects. This technology is in the early stage of development but its feasibility in treating ocular pathologies has been demonstrated in various preclinical in vitro and in vivo models as well as in the recruitment of patients for a clinical trial to treat refractory viral keratitis (Amador et al., 2021; Mohan et al., 2021b). Gene editing was successfully done for both anterior and posterior regions of the eye in an experimental model using mostly viral vectors (Amador et al., 2021). The CRISPR/Cas9 system was used to study aniridia-related keratopathy caused by a heterozygous PAX6 gene mutation causing congenital aniridia (Roux et al., 2018). The addition of recombinant PAX6 protein improved the therapeutic effect for congenital aniridia. CRISPR/Cas9-based gene therapy was used to promote wound healing and corneal endothelial regeneration using in vivo and in vitro models (Chang et al., 2018).
7.2. Stem cell therapy
Stem cells are undifferentiated cells present in the embryonic, fetal, and adult stages of life with an ability to differentiate in desired cell types to build various tissues and organs. Stem cells have the characteristics of self-renewal (proliferate extensively), clonality (generally arising from a single cell), and potency (the ability to differentiate into different cell types). Stem cells are classified as totipotent (zygote), pluripotent (embryonic stem cells and induced pluripotent stem cells (iPSCs), multipotent mesenchymal stem cells (MSCs), and oligopotent (able to form two or more mature cell types within a tissue) (Miotti et al., 2021). Embryonic stem cells come from three to five days old embryos. Adult stem cells are present in most of the organs, such as bone marrow and fat. Adult stem cells have limited proliferative potential and differentiation. Stem cell therapy is also called regenerative medicine. Stem cells have been explored for tissue regeneration in many tissues and organs, including the eye, due to their proliferative potential and plasticity (Miotti et al., 2021). In recent years significant progress has been made in using stem cells for the treatment of corneal blindness (Kumar et al., 2021). Several stem cell therapies to treat various diseases in humans have been approved by the FDA. There are several stem cell therapy clinical trials to treat corneal disorders, including stromal regeneration using iPSCs, ASCs, UC-MSCs etc. (Miotti et al., 2021).
The corneal limbal stem cells (LSC) are derived from the corneal periphery at the limbal region beneath the palisades of Vogt. Injuries such as chemical burns, infections, SJS etc. can damage the stem cell pool at the limbus causing limbal stem cell deficiency (LSCD) (Jackson et al., 2020). This compromises the corneal epithelium, leading to loss of corneal homeostasis and conjunctival ingrowth. The persistent epithelial defects in LSCD can cause scarring and vision loss. LSC-based treatments for LSCD have been approved in many countries over the past decade (Jackson et al., 2020). LSC transplants lead to re-epithelialization of the corneas, usually within 2 weeks, improving visual clarity and preventing scars. These procedures are broadly divided into CLET (cultured limbal epithelial transplantation) and SLET (simple limbal epithelial transplantation). In CLET, limbal biopsies from healthy eyes are grown on an amniotic membrane (AM) to produce limbal cell sheets which are transplanted into the affected corneas (Pellegrini et al., 2009). In SLET, a limbal biopsy from the healthy eye is divided into smaller chunks and distributed on AM glued to the affected cornea (Shanbhag et al., 2019). The cell outgrowth from the explant pieces re-epithelializes the cornea.
MSCs have been evaluated for corneal engineering. MSCs can differentiate into keratocytes, epithelial and endothelial cells (Ghiasi et al., 2021). MSCs have the potential for the treatment of keratitis, reducing inflammation, and inhibition of neovascularization, and therefore, these cells can be used as a novel cellular therapy for corneal repairing (Ghiasi et al., 2021). Corneal epithelium refreshes in 7–14 days with new cells originating from the LSC. These LSCs are the reservoir cells in corneal tissue to replenish the lost cells.
Stem cells produce/secrete cytokines and growth factors in a tissue microenvironment. Secretomes, like extracellular vehicles (EVs), can regulate wide-ranging functional responses comprising of soluble proteins, growth factors, cytokines, chemokines, microRNAs, bioactive lipids, and all types of secretions released from a cell in sizes ranging from 40 to 1000 nm, including exosomes (40–150 nm) and microvesicles (150–600 nm). Stem cell EVs and exosomes for ocular tissue regeneration have been reported (Kumar et al., 2021). CSSC administration is reported to reduce corneal opacity and inflammation, restore stromal ultrastructure, and improve vision (Kumar et al., 2021). Human CSSC can transform into keratocytes with the acquisition of keratocan and keratin sulfate in vitro. These in vitro generated cells can be used for corneal bioengineering purposes. Human ASC could be another source for corneal stromal regeneration as they can differentiate into keratocytes in culture. Autologous ASC usage showed some success in keratoconus patients improved visual acuity, corneal transparency, collagen formation, and increased corneal thickness without major adverse effects (Kumar et al., 2021). The previous report indicates that human limbal biopsy-derived stromal cells (LBSCs) or human limbus-derived stromal/mesenchymal stem cells (hLMSC) have the potential to differentiate into keratocytes, stroma-like tissue, and prevent corneal scarring in mouse cornea (Basu et al., 2014). In another study, investigators evaluated the potential of MSCs to suppress the allogenic immune response in releasing HGF using in vivo mouse model. They found that topical administration of HGF suppresses immune cells and inflammatory response in the grafted tissue suggesting HGF-based therapeutic options (Mittal et al., 2019). A recent review reported the potential of limbal epithelial stem cells and MSCs for stem cell therapy for corneal regeneration (Kumar et al., 2021; Shukla et al., 2020). Stem cells have the potential for stromal regeneration and to treat corneal diseases, however, more research is needed to advance this technology.
7.3. Tissue engineering
Tissue engineering is an appealing approach to repair, restore, and replace different types of tissues in the body. It involves the embedding of cells on scaffolds to form a viable tissue and/or surface on which cells can grow. Host-graft interactions are vital for the proper integration and long-term functionality of engineered transplants (Mijanovic et al., 2021). The main challenge with this technology is the incompetence of biomaterials and scaffolds to connect with surrounding tissues, which limits its application to correct damaged corneal tissues. Advances in nanotechnology may offer some solutions to this problem. This technology has been tried to regenerate corneal stromal tissues. Growth factors such as FGF2 can improve corneal scaffolds and enhance the success of tissue engineering (Matthyssen et al., 2018). Silk fibrin is used to make the scaffolds to construct the cornea (Matthyssen et al., 2018). Corneal stromal tissue engineering includes using gelatin, polymers, collagen-like peptides, decellularized cornea, silk, and cell-based alternatives (Matthyssen et al., 2018). Collagen-based corneal tissue engineering includes collagen type 1, collagen type III, as well as multiple collagen types in various forms, different species, crosslinking and various cells in vitro and in vivo applications (Matthyssen et al., 2018). Currently, a great deal of research is focused on creating a full-thickness engineered cornea with epithelium, stroma, and endothelial layers using primary cells and cell lines are underway and preclinical testing has shown promise of tissue engineering technology for future clinical use (Guerin et al., 2021).
7.4. Xenotransplantation
The implantation or infusion of live cells, tissues, or organs from an animal source into a human has also been tested. However, the fate of xenotransplantation is unknown due to safety and long-term viability concerns. Decellularized porcine stromal grafts were performed in human patients in China suffering from corneal blindness from fungal and viral infections. However, the future of this ambitious treatment remains unknown because of multiple complications exhibited by recipients during postoperative care (Zhang et al., 2015; Zheng et al., 2019). Because of the incompatibility of the xenogenic tissues, decellularization is necessary to reduce immunological response. In addition to porcine corneas, fish scale-derived stromal scaffolds for the treatment of corneal perforation disorders have also been tested in animal models (Chen et al., 2015; van Essen et al., 2013).
7.5. Bioengineered stromal equivalents and 3D bioprinting
The bioengineered stromal equivalents offer many advantages. These include reduced immune attack, no decellularization requirement, and commercial manufacturing. The acellular recombinant human collagen-based scaffold is termed as ‘biosynthetic’ corneal stroma. This type of biosynthetic cornea survived for years in patients with nerve regeneration and without major inflammatory reaction or rejection (Fagerholm et al., 2009; Lagali, 2020). To improve the usefulness of biosynthetic scaffolds, the crosslinking system is used. Corneal collagen crosslinking (CXL) enhancing tensile strength and rigidity of the cornea is used to treat corneal diseases such as keratoconus or corneal ectasia (Greenstein and Hersh, 2021; Pasha et al., 2021; Vohra et al., 2021b; Wu et al., 2021). Another option explored is a plastic compressed collagen matrix for temporary use to treat conditions including destructive ulcers.
Cell populated engineered scaffolds are also tested to treat severely dysfunctional cornea. In this modality, the fibrin-agarose scaffold made of human plasma is tested for treating stromal disorders such as stromal thinning. The CelCORE technology has been developed which involves the application of scaffolds consisting of gel-like viscous substance followed by hardening with photopolymerization has been developed (Shirzaei Sani et al., 2019). This technology offers suture-less repair of the severely damaged cornea. A recent study demonstrated a simple approach that decellularized corneal matrix hydrogel from the animal to treat corneal scars in the injured cornea (Chameettachal et al., 2021). Another study showed that the inclusion of corneal tissue-derived ECM microparticles in fibrin hydrogels is a safe therapeutic option to treat superficial epithelial and anterior stromal injuries (Chandru et al., 2021). 3D bioprinting is an emerging method for the fabrication of biological grade cornea. A bioprinter can combine different biomaterials such as keratocytes, collagens, stem cells, plasma, and other molecules in the matrix with high spatial resolution. A major advantage of 3D printed scaffolds is the delivery of precision medicine based on fulfilling patient-specific needs (Isaacson et al., 2018). These techniques are presently in preclinical evaluations.
8. Conclusions and future directions
Corneal stromal pathologies including scarring, inflammation, neovascularization, keratoconus, and neurodegeneration remain a major challenge and leading cause of global blindness. Current non-surgical therapies allow reasonable resolution of mild-moderate corneal blindness. Surgical interventions such as penetrating anterior and posterior lamellar keratoplasty and full-thickness corneal transplantation are the standard of care currently to restore corneal function and vision in patients. However, many factors restrict the wide use of corneal transplantation surgeries including a limited supply of good quality donor corneas for grafting. Thus, more research directed towards an understanding of mechanisms and pathways promoting stromal repair/regeneration and restoring transparency is needed. In particular, the identification and clinical testing of predisposing/risk predicting molecular factors could help in patient selection for refractive surgery, a precision medicine approach that could significantly reduce the associated morbidity. Additionally, research defining the role of infiltrating inflammatory cells in the cornea after injury influencing stromal function and healing is desired. Corneal epithelium can regenerate after injury, but stroma struggles to regenerate. Stromal repair/regeneration is regulated by a complex cascade of events orchestrated by both local factors and systemic influences regulating each other. These processes include but are not limited to keratocyte apoptosis, quiescent stromal keratocyte activation, proliferation, migration and differentiation, myofibroblast formation, ECM remodeling, secretomes/exosomes, epigenetics, TGF-β and other signaling pathways, and collagen fibrillogenesis. Studies on corneal wound healing, especially in the last two decades, unveils the role of many new genes, factors, pathways, and mechanisms that influence stromal repair and regeneration after injury. In particular, the recent evidence of the interplay between autophagy, immune activation and the well-known profibrotic mechanisms lay the foundation for combinational therapies which may have a much broader scope of managing stromal fibrosis of diverse etiologies. A significant repair and restoration of stroma and corneal transparency were shown by the delivery of two genes or eye drops containing multiple drugs/molecules targeting more than one mechanism in vivo in the preclinical rabbit model. Nonetheless, a battery of additional in vivo animal and human cornea ex vivo organ culture studies is essential to shedding more light on the promise of such novel corneal regenerative therapeutics. Gene therapy continues to develop and rapidly advance to successfully prevent, treat, and cure corneal blindness and has a high potential for human application considering FDA has already approved gene therapy for retinal disorders. The development of other newer therapeutic strategies includes improved laser procedures, limbal stem cells, stromal stem cells, embryonic stem cells, induced pluripotent stem cells, nanomedicine, exosomes, neural regeneration, stromal equivalents, artificial corneas, 3D bioprinting, bioengineered stromal scaffolds, tissue adhesives, and corneal sensory nerve regeneration. However, as new data evolves, modulation of the wound-specific cellular immune response to control the stromal fibrotic processes is desired. Further, understanding the immune cell biology of wound healing and graft rejection in human patients may be critical in devising novel therapeutic strategies to manage fibrosis clinically. Translating such discoveries for clinical use remains the primary hurdle that needs to be overcome not only by novel therapeutics but also by the repurposing of established drugs. Ongoing research solving challenges and defining the mechanisms of these approaches will lead to effective methods to repair and restore stromal function, corneal transparency, and ultimately their clinical applications in humans to treat corneal blindness.
Acknowledgments/Funding
The work presented in this article was primarily supported by the National Institutes of Health 5R01EY017294, 5R01EY030774, 1U01EY031650, and 1R21EY032742 grants, Bethesda, Maryland, USA, the United States Department of Veterans Health Affairs 1I01BX00357 and IK6BX005646 grants, Washington D.C., USA, and the University of Missouri Ruth M. Kraeuchi Missouri Endowed Chair Ophthalmology Fund, Columbia, Missouri, USA.
List of abbreviations
- AAV
Adeno-associated virus
- AMG
Amniotic membrane graft
- ASC
Adipose-derived stem cells
- α-SMA
Alpha-smooth muscle actin
- bFGF
Basic fibroblast growth factor
- KCa3.1
Calmodulin/calcium-activated K+ channels
- CCFs
Canine corneal fibroblasts
- CCL2
Chemokine (C–C motif) ligand 2
- CMFs
Corneal myofibroblasts
- COVID-19
Coronavirus disease 2019
- CSSC
Corneal stromal stem cells
- CXL
Corneal collagen crosslinking
- DAMPs
Damage-associated molecular patterns
- DBM
Descemet’s basement membrane
- dECM
Decellularized extracellular matrix
- DNA
Deoxyribonucleic acid
- EBM
Epithelial basement membrane
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- ESC
Embryonic stem cells
- EVs
Extracellular vesicles
- FGF
Fibroblast growth factor
- GAG
Glycosaminoglycan
- GDNF
Glial cell-derived neurotrophic factor
- GFP
Green fluorescent protein
- GFS
Glaucoma filtration surgery
- GNP
Gold nanoparticles
- HDACi
Histone deacetylase inhibitor
- HGF
Hepatocyte growth factor
- HSF
Human corneal fibroblast
- IL
Interleukin
- iPSC
Induced pluripotent stem cells
- JNK
c-Jun N-terminal kinase
- JAK-STAT
Janus tyrosine kinase-Signal transducer and activator of transcription
- KCa3.1
Calmodulin/calcium-activated K+ channels 3.1
- KGF
Keratinocyte growth factor
- LASIK
Laser-assisted in situ keratomileusis
- LSC
Limbal stem cells
- MAPKs
Mitogen-activated protein kinase
- MMPs
Matrix metalloproteases
- MPO
Myeloperoxidase
- MSCs
Mesenchymal stem cells
- NETs
Neutrophil extracellular traps
- NF-kB
Nuclear factor-kappaB
- NGF
Nerve growth factor
- NPs
Nanoparticles
- MNPs
Magnetic nanoparticles
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PAMPS
Pathogen-associated molecular patterns
- PDGF
Platelet-derived growth factor
- PEDF
Pigment epithelium-derived factor
- PEI
Polyethylenimine
- PEI2-GNP
Polyethylenimine-conjugated gold nanoparticle
- PF
Peritoneal fibrosis
- PK
Penetrating keratoplasty
- PRK
Photorefractive keratectomy
- RCES
Recurrent corneal erosion syndrome
- ROS
Reactive oxygen species
- SAHA
Suberoylanilide hydroxamic acid
- shRNA
Short hairpin ribonucleic acid
- sTGFβRII
Soluble transforming growth factor-β type II receptor
- SLRP
Small leucine-rich proteoglycans
- Smad
Suppressor of mothers against decapentaplegic
- SMILE
Small incision lenticule extraction
- SP
Substance P
- TALENs
Transcription activator-like effector nucleases
- TBI
Traumatic brain injury
- TED
Turbo eye drops
- TEM
Transmission Electron Microscopy
- TGF-β
Transforming growth factor beta
- TJ
Tight junction
- TNF-α
Tumor necrosis factor-alpha
- TSA
Trichostatin A
- TRAM-34
Triarylmethane-34
- UV
Ultraviolet
- VEGF
Vascular endothelial growth factor
- WHO
World Health Organization
- ZNFs
Zinc finger nucleases
Footnotes
CRediT authorship contribution statement
Rajiv R. Mohan: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, review & editing, generating graphical abstract and . Duraisamy Kempuraj: Writing and editing. Sharon D’Souza: MBBS, MD: Assisting in clinical part of original draft, Resources, Figure . Arkasubhra Ghosh: PhD: Assisting in writing inflammation aspect of original draft, review & editing, Resources, and .
Declaration of competing interest
None.
References
- Agarwal S, Srinivasan B, Gupta R, Iyer G, 2020. Allogenic simple limbal epithelial transplantation versus amniotic membrane grafting in the early management of severe-grade ocular chemical injuries-A retrospective comparative study. Am. J. Ophthalmol 217, 297–304. [DOI] [PubMed] [Google Scholar]
- Aghaji AE, Duke R, Aghaji UCW, 2019. Inequitable coverage of vitamin A supplementation in Nigeria and implications for childhood blindness. BMC Publ. Health 19, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F, House RJ, Feldman BH, 2015. Corneal abrasions and corneal foreign bodies. Prim Care 42, 363–375. [DOI] [PubMed] [Google Scholar]
- Alio Del Barrio JL, Arnalich-Montiel F, De Miguel MP, El Zarif M, Alio JL, 2021a. Corneal stroma regeneration: preclinical studies. Exp. Eye Res 202, 108314. [DOI] [PubMed] [Google Scholar]
- Alio Del Barrio JL, Bhogal M, Ang M, Ziaei M, Robbie S, Montesel A, Gore DM, Mehta JS, Alio JL, 2021b. Corneal transplantation after failed grafts: options and outcomes. Surv. Ophthalmol 66, 20–40. [DOI] [PubMed] [Google Scholar]
- Allan B, 1999. Artificial corneas. BMJ 318, 821–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador C, Shah R, Ghiam S, Kramerov AA, Ljubimov AV, 2021. Gene therapy in the anterior eye segment. Curr. Gene Ther 22, 104–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K, El-Sheikh A, Newson T, 2004. Application of structural analysis to the mechanical behaviour of the cornea. J. R. Soc. Interface 1, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanthan G, Gupta S, Fink MK, Hesemann NP, Bowles DK, McDaniel LM, Muhammad M, Mohan RR, 2018. KCa3.1 ion channel: a novel therapeutic target for corneal fibrosis. PLoS One 13, e0192145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanthan G, Sharma A, Waggoner M, Hamm CW, Gupta S, Hesemann NP, Mohan RR, 2017. Efficacy and safety comparison between suberoylanilide hydroxamic acid and mitomycin C in reducing the risk of corneal haze after PRK treatment in vivo. J. Refract. Surg 33, 834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz-Marquez E, Katsanos A, Kozobolis VP, Konstas AGP, Teus MA, 2019. A critical overview of the biological effects of mitomycin C application on the cornea following refractive surgery. Adv. Ther 36, 786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi P, Singh P, Raj A, 2021. Surgical management and recent advances in chemical injury: a 5-year review. Semin. Ophthalmol 37, 49–56. [DOI] [PubMed] [Google Scholar]
- Balne PK, Gupta S, Zhang J, Bristow D, Faubion M, Heil SD, Sinha PR, Green SL, Iozzo RV, Mohan RR, 2021. The functional role of decorin in corneal neovascularization in vivo. Exp. Eye Res 207, 108610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balne PK, Sinha NR, Hofmann AC, Martin LM, Mohan RR, 2020. Characterization of hydrogen sulfide toxicity to human corneal stromal fibroblasts. Ann. N. Y. Acad. Sci 1480, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran-Rafii A, Eslani M, Haq Z, Shirzadeh E, Huvard MJ, Djalilian AR, 2017. Current and upcoming therapies for ocular surface chemical injuries. Ocul. Surf 15, 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JT, Wilson BS, Gordon MO, Rah MJ, Riley C, Kollbaum PS, Zadnik K, Group CS, 2006. Estimation of the incidence and factors predictive of corneal scarring in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Cornea 25, 16–25. [DOI] [PubMed] [Google Scholar]
- Barrientez B, Nicholas SE, Whelchel A, Sharif R, Hjortdal J, Karamichos D, 2019. Corneal injury: clinical and molecular aspects. Exp. Eye Res 186, 107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL, 2014. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci. Transl. Med. 6, 266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Vaddavalli PK, Ramappa M, Shah S, Murthy SI, Sangwan VS, 2011. Intracameral perfluoropropane gas in the treatment of acute corneal hydrops. Ophthalmology 118, 934–939. [DOI] [PubMed] [Google Scholar]
- Bielory L, Kempuraj D, Theoharides T, 2002. Topical immunopharmacology of ocular allergies. Curr. Opin. Allergy Clin. Immunol 2, 435–445. [DOI] [PubMed] [Google Scholar]
- Bosiack AP, Giuliano EA, Gupta R, Mohan RR, 2012. Efficacy and safety of suberoylanilide hydroxamic acid (Vorinostat) in the treatment of canine corneal fibrosis. Vet. Ophthalmol 15, 307–314. [DOI] [PubMed] [Google Scholar]
- Brown CT, Lin P, Walsh MT, Gantz D, Nugent MA, Trinkaus-Randall V, 2002. Extraction and purification of decorin from corneal stroma retain structure and biological activity. Protein Expr. Purif 25, 389–399. [DOI] [PubMed] [Google Scholar]
- Brunette I, Roberts CJ, Vidal F, Harissi-Dagher M, Lachaine J, Sheardown H, Durr GM, Proulx S, Griffith M, 2017. Alternatives to eye bank native tissue for corneal stromal replacement. Prog. Retin. Eye Res 59, 97–130. [DOI] [PubMed] [Google Scholar]
- Burton MJ, 2009. Prevention, treatment and rehabilitation. Community Eye Health 22, 33–35. [PMC free article] [PubMed] [Google Scholar]
- Buss DG, Giuliano E, Sharma A, Mohan RR, 2010. Gene delivery in the equine cornea: a novel therapeutic strategy. Vet. Ophthalmol 13, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M, Nielsen S, Szerenyi K, Garbus JJ, McDonnell PJ, 1993. Clinical follow-up of phototherapeutic keratectomy for treatment of corneal opacities. Am. J. Ophthalmol 115, 433–440. [DOI] [PubMed] [Google Scholar]
- Carniciu AL, Fazzari MJ, Tabibian P, Batta P, Gentile RC, Grendell JH, Braithwaite CE, Barzideh N, 2017. Corneal abrasion following anaesthesia for non-ocular surgical procedures: a case-controlled study. J. Perioperat. Pract 27, 247–253. [DOI] [PubMed] [Google Scholar]
- Catala P, Thuret G, Skottman H, Mehta JS, Parekh M, Ni Dhubhghaill S, Collin RWJ, Nuijts R, Ferrari S, LaPointe VLS, Dickman MM, 2021. Approaches for corneal endothelium regenerative medicine. Prog. Retin. Eye Res 100987. [DOI] [PubMed] [Google Scholar]
- Chamberlain WD, 2019. Femtosecond laser-assisted deep anterior lamellar keratoplasty. Curr. Opin. Ophthalmol 30, 256–263. [DOI] [PubMed] [Google Scholar]
- Chameettachal S, Prasad D, Parekh Y, Basu S, Singh V, Bokara KK, Pati F, 2021. Prevention of corneal myofibroblastic differentiation in vitro using a biomimetic ECM hydrogel for corneal tissue regeneration. ACS Appl. Bio Mater 4, 533–544. [DOI] [PubMed] [Google Scholar]
- Chandru A, Agrawal P, Ojha SK, Selvakumar K, Shiva VK, Gharat T, Selvam S, Thomas MB, Damala M, Prasad D, Basu S, Bhowmick T, Sangwan VS, Singh V, 2021. Human cadaveric donor cornea derived extra cellular matrix microparticles for minimally invasive healing/regeneration of corneal wounds. Biomolecules 11, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YK, Hwang JS, Chung TY, Shin YJ, 2018. SOX2 activation using CRISPR/dCas9 promotes wound healing in corneal endothelial cells. Stem Cell. 36, 1851–1862. [DOI] [PubMed] [Google Scholar]
- Chaudhary K, Moore H, Tandon A, Gupta S, Khanna R, Mohan RR, 2014. Nanotechnology and adeno-associated virus-based decorin gene therapy ameliorates peritoneal fibrosis. Am. J. Physiol. Ren. Physiol 307, F777–F782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Telinius N, Lin HT, Huang MC, Lin CC, Chou CH, Hjortdal J, 2015. Use of fish scale-derived BioCornea to seal full-thickness corneal perforations in pig models. PLoS One 10, e0143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Mittal SK, Elbasiony E, Chauhan SK, 2020. Activation of ocular surface mast cells promotes corneal neovascularization. Ocul. Surf 18, 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Kang HG, Lee CH, Yeo A, Noh HM, Gu N, Kim MJ, Song JS, Kim HC, Lee HK, 2017. Langerhans cells prevent subbasal nerve damage and upregulate neurotrophic factors in dry eye disease. PLoS One 12, e0176153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin C, 2021. Corneal dystrophies: pathophysiological, genetic, clinical, and therapeutic considerations. Rom J. Ophthalmol 65, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland R, Natalie A, 2013. Principles and Practive Of Cornea. Jaypee Brothers Medical Publishers (P) Ltd. [Google Scholar]
- Coursey TG, Bian F, Zaheer M, Pflugfelder SC, Volpe EA, de Paiva CS, 2017. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunol. 10, 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Hos D, 2021. Cutting edge: novel treatment options targeting corneal neovascularization to improve high-risk corneal graft survival. Cornea 40, 1512–1518. [DOI] [PubMed] [Google Scholar]
- D’Souza S, Nair AP, Sahu GR, Vaidya T, Shetty R, Khamar P, Mullick R, Gupta S, Dickman MM, Nuijts R, Mohan RR, Ghosh A, Sethu S, 2021. Keratoconus patients exhibit a distinct ocular surface immune cell and inflammatory profile. Sci. Rep 11, 20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervenis P, VasilakisPh DP, Stathi T, Giannoulakos G, Moula K, Dervenis N, Praidou A, Rempapis I, 2021. Acute corneal melting one week after an uncomplicated cataract surgery in a patient who previously underwent eyelid radiation and with undiagnosed rheumatoid arthritis: a case report. Arq. Bras. Oftalmol 84, 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh R, Stevenson LJ, Vajpayee R, 2020. Management of corneal perforations: an update. Indian J. Ophthalmol 68, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Alvisi G, Trevisan M, Ferrari S, Masi G, Nespeca P, Ghassabian H, Ponzin D, Palu G, 2019. New frontiers of corneal gene therapy. Hum. Gene Ther 30, 923–945. [DOI] [PubMed] [Google Scholar]
- Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana R, 2017. Management of high-risk corneal transplantation. Surv. Ophthalmol 62, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KS, Giuliano EA, Sharm A, Mohan RR, 2014a. Suberoylanilide hydroxamic acid (vorinostat): its role on equine corneal fibrosis and matrix metalloproteinase activity. Vet. Ophthalmol 17 (Suppl. 1), 61–68. [DOI] [PubMed] [Google Scholar]
- Donnelly KS, Giuliano EA, Sharma A, Tandon A, Rodier JT, Mohan RR, 2014b. Decorin-PEI nanoconstruct attenuates equine corneal fibroblast differentiation. Vet. Ophthalmol 17, 162–169. [DOI] [PubMed] [Google Scholar]
- Dua HS, Ting DSJ, Al Saadi A, Said DG, 2020. Chemical eye injury: pathophysiology, assessment and management. Eye 34, 2001–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elieh Ali Komi D, Rambasek T, Bielory L, 2018. Clinical implications of mast cell involvement in allergic conjunctivitis. Allergy 73, 528–539. [DOI] [PubMed] [Google Scholar]
- Elieh Ali Komi D, Wohrl S, Bielory L, 2020. Mast cell biology at molecular level: a comprehensive review. Clin. Rev. Allergy Immunol 58, 342–365. [DOI] [PubMed] [Google Scholar]
- Espana EM, Birk DE, 2020. Composition, structure and function of the corneal stroma. Exp. Eye Res 198, 108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm P, Lagali NS, Carlsson DJ, Merrett K, Griffith M, 2009. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. Clin. Transl. Sci 2, 162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MK, Giuliano EA, Tandon A, Mohan RR, 2015. Therapeutic potential of Pirfenidone for treating equine corneal scarring. Vet. Ophthalmol 18, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan G, Velez T, Gu W, Singman E, 2020. The relationship between severe visual acuity loss, traumatic brain injuries, and ocular injuries in American service members from 2001 to 2015. Mil. Med 185, e1576–e1583. [DOI] [PubMed] [Google Scholar]
- Frey N, Jossi J, Bodmer M, Bircher A, Jick SS, Meier CR, Spoendlin J, 2017. The epidemiology of stevens-johnson syndrome and toxic epidermal necrolysis in the UK. J. Invest. Dermatol 137, 1240–1247. [DOI] [PubMed] [Google Scholar]
- Frick KD, Singman EL, 2019. Cost of military eye injury and vision impairment related to traumatic brain injury: 2001-2017. Mil. Med 184, e338–e343. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Giuliano EA, Sinha NR, Mohan RR, 2021. Ocular toxicity of mustard gas: a concise review. Toxicol. Lett 343, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuest M, Yam GH, Peh GS, Mehta JS, 2016. Advances in corneal cell therapy. Regen. Med 11, 601–615. [DOI] [PubMed] [Google Scholar]
- Fullwood NJ, 2004. Collagen fibril orientation and corneal curvature. Structure 12, 169–170. [DOI] [PubMed] [Google Scholar]
- Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G, 2016. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 134, 167–173. [DOI] [PubMed] [Google Scholar]
- Gao N, Yin J, Yoon GS, Mi QS, Yu FS, 2011. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am. J. Pathol 179, 2243–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova RM, Meyer KJ, Anderson MG, Harper MM, Pieper AA, 2018. Neprilysin inhibition promotes corneal wound healing. Sci. Rep 8, 14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi M, Jadidi K, Hashemi M, Zare H, Salimi A, Aghamollaei H, 2021. Application of mesenchymal stem cells in corneal regeneration. Tissue Cell 73, 101600. [DOI] [PubMed] [Google Scholar]
- Gong Q, Zhang S, Jiang L, Lin M, Xu Z, Yu Y, Wang Q, Lu F, Hu L, 2021. The effect of nerve growth factor on corneal nerve regeneration and dry eye after LASIK. Exp. Eye Res 203, 108428. [DOI] [PubMed] [Google Scholar]
- Greenstein SA, Hersh PS, 2021. Corneal crosslinking for progressive keratoconus and corneal ectasia: summary of US multicenter and subgroup clinical trials. Transl. Vis. Sci. Technol 10, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronkiewicz KM, Giuliano EA, Kuroki K, Bunyak F, Sharma A, Teixeira LB, Hamm CW, Mohan RR, 2016a. Development of a novel in vivo corneal fibrosis model in the dog. Exp. Eye Res 143, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronkiewicz KM, Giuliano EA, Sharma A, Mohan RR, 2016b. Molecular mechanisms of suberoylanilide hydroxamic acid in the inhibition of TGF-beta1-mediated canine corneal fibrosis. Vet. Ophthalmol 19, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin LP, Le-Bel G, Desjardins P, Couture C, Gillard E, Boisselier E, Bazin R, Germain L, Guerin SL, 2021. The human tissue-engineered cornea (hTEC): recent progress. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Buyank F, Sinha NR, Grant DG, Sinha PR, Iozzo RV, Chaurasia SS, Mohan RR, 2022. Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo. Exp. Eye Res 108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Fink MK, Ghosh A, Tripathi R, Sinha PR, Sharma A, Hesemann NP, Chaurasia SS, Giuliano EA, Mohan RR, 2018. Novel combination BMP7 and HGF gene therapy instigates selective myofibroblast apoptosis and reduces corneal haze in vivo. Invest. Ophthalmol. Vis. Sci 59, 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Fink MK, Martin LM, Sinha PR, Rodier JT, Sinha NR, Hesemann NP, Chaurasia SS, Mohan RR, 2020a. A rabbit model for evaluating ocular damage from acrolein toxicity in vivo. Ann. N. Y. Acad. Sci 1480, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Martin LM, Sinha NR, Smith KE, Sinha PR, Dailey EM, Hesemann NP, Mohan RR, 2020b. Role of inhibitor of differentiation 3 gene in cellular differentiation of human corneal stromal fibroblasts. Mol. Vis 26, 742–756. [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR, 2017. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS One 12, e0172928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Sinha NR, Martin LM, Keele LM, Sinha PR, Rodier JT, Landreneau JR, Hesemann NP, Mohan RR, 2021. Long-term safety and tolerability of BMP7 and HGF gene overexpression in rabbit cornea. Transl. Vis. Sci. Technol 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Dana MR, 2007. Corneal antigen-presenting cells. Chem. Immunol. Allergy 92, 58–70. [DOI] [PubMed] [Google Scholar]
- Hassan OM, Farooq AV, Soin K, Djalilian AR, Hou JH, 2017. Management of corneal scarring secondary to herpes zoster keratitis. Cornea 36, 1018–1023. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE, 2010. The molecular basis of corneal transparency. Exp. Eye Res 91, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bazan HE, 2016. Neuroanatomy and neurochemistry of mouse cornea. Invest. Ophthalmol. Vis. Sci 57, 664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty DM, Hermes SM, Morgan MM, Aicher SA, 2018. Acute hyperalgesia and delayed dry eye after corneal abrasion injury. Pain Rep. 3, e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch ML, Conatser LM, Smith SM, Salmon JH, Wu J, Buglak NE, Davis R, Gilger BC, 2017. AAV vector-meditated expression of HLA-G reduces injury-induced corneal vascularization, immune cell infiltration, and fibrosis. Sci. Rep 7, 17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland G, Pandit A, Sanchez-Abella L, Haiek A, Loinaz I, Dupin D, Gonzalez M, Larra E, Bidaguren A, Lagali N, Moloney EB, Ritter T, 2021. Artificial cornea: past, current, and future directions. Front. Med 8, 770780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hos D, Matthaei M, Bock F, Maruyama K, Notara M, Clahsen T, Hou Y, Le VNH, Salabarria AC, Horstmann J, Bachmann BO, Cursiefen C, 2019. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res 73, 100768. [DOI] [PubMed] [Google Scholar]
- Huang YX, Li QH, 2007. An active artificial cornea with the function of inducing new corneal tissue generation in vivo-a new approach to corneal tissue engineering. Biomed. Mater 2, S121–S125. [DOI] [PubMed] [Google Scholar]
- Isaacson A, Swioklo S, Connon CJ, 2018. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res 173, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CJ, Myklebust Erno IT, Ringstad H, Tonseth KA, Dartt DA, Utheim TP, 2020. Simple limbal epithelial transplantation: current status and future perspectives. Stem Cells Transl. Med 9, 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Sharma N, Basu S, Iyer G, Ueta M, Sotozono C, Kannabiran C, Rathi VM, Gupta N, Kinoshita S, Gomes JA, Chodosh J, Sangwan VS, 2016. Stevens-Johnson syndrome: the role of an ophthalmologist. Surv. Ophthalmol 61, 369–399. [DOI] [PubMed] [Google Scholar]
- Jeon KI, Hindman HB, Bubel T, McDaniel T, DeMagistris M, Callan C, Huxlin KR, 2018. Corneal myofibroblasts inhibit regenerating nerves during wound healing. Sci. Rep 8, 12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD, 1997. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea 16, 177–187. [PubMed] [Google Scholar]
- Jhanji V, Mehta JS, Sharma N, Sharma B, Vajpayee RB, 2012. Targeted corneal transplantation. Curr. Opin. Ophthalmol 23, 324–329. [DOI] [PubMed] [Google Scholar]
- Jhanji V, Young AL, Mehta JS, Sharma N, Agarwal T, Vajpayee RB, 2011. Management of corneal perforation. Surv. Ophthalmol 56, 522–538. [DOI] [PubMed] [Google Scholar]
- Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana R, 2009. Anti-angiogenesis effect of the novel anti-inflammatory and proresolving lipid mediators. Invest. Ophthalmol. Vis. Sci 50, 4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil S, Mohan RR, 2021. Corneal stromal wound healing: major regulators and therapeutic targets. Ocul. Surf 19, 290–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu LN, Ciolino JB, 2021. Nerve growth factor as an ocular therapy: applications, challenges, and future directions. Semin. Ophthalmol 36, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar JR, Sun X, Punj V, Sriramoju B, Mohan RR, Zhou SF, Chauhan A, Kanwar RK, 2012. Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal. Nanomedicine 8, 399–414. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Escandon P, Vasini B, Nicholas SE, Van L, Dang DH, Cunningham RL, Riaz KM, 2021. Anterior pituitary, sex hormones, and keratoconus: beyond traditional targets. Prog. Retin. Eye Res 101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kate A, Vyas S, Bafna RK, Sharma N, Basu S, 2021. Tenon’s patch graft: a review of indications, surgical technique, outcomes and complications. Semin. Ophthalmol 1–9. [DOI] [PubMed] [Google Scholar]
- Keating AM, Jacobs DS, 2011. Anti-VEGF treatment of corneal neovascularization. Ocul. Surf 9, 227–237. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Huang M, Kandere K, Boucher W, Letourneau R, Jeudy S, Fitzgerald K, Spear K, Athanasiou A, Theoharides TC, 2002. Azelastine is more potent than olopatadine n inhibiting interleukin-6 and tryptase release from human umbilical cord blood-derived cultured mast cells. Ann. Allergy Asthma Immunol 88, 501–506. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS, Zaheer A, 2019. Mast cells in stress, pain, bloodbrain barrier, neuroinflammation and alzheimer’s disease. Front. Cell. Neurosci 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A, 2016. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 1, 1003. [PMC free article] [PubMed] [Google Scholar]
- Khamar P, Nair AP, Shetty R, Vaidya T, Subramani M, Ponnalagu M, Dhamodaran K, D’Souza S, Ghosh A, Pahuja N, Deshmukh R, Ahuja P, Sainani K, Nuijts R, Das D, Ghosh A, Sethu S, 2019. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest. Ophthalmol. Vis. Sci 60, 2532–2542. [DOI] [PubMed] [Google Scholar]
- Kim EC, Kim TK, Park SH, Kim MS, 2012. The wound healing effects of vitamin A eye drops after a corneal alkali burn in rats. Acta Ophthalmol. 90, e540–546. [DOI] [PubMed] [Google Scholar]
- Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, Blomquist PH, Bouchard CS, Dart JK, Gai X, Gomes JA, Gregory DG, Iyer G, Jacobs DS, Johnson AJ, Kinoshita S, Mantagos IS, Mehta JS, Perez VL, Pflugfelder SC, Sangwan VS, Sippel KC, Sotozono C, Srinivasan B, Tan DT, Tandon R, Tseng SC, Ueta M, Chodosh J, 2016a. Acute and chronic ophthalmic involvement in stevens-johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul. Surf 14, 168–188. [DOI] [PubMed] [Google Scholar]
- Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, Blomquist PH, Bouchard CS, Dart JK, Gai X, Gomes JA, Gregory DG, Iyer G, Jacobs DS, Johnson AJ, Kinoshita S, Mantagos IS, Mehta JS, Perez VL, Pflugfelder SC, Sangwan VS, Sippel KC, Sotozono C, Srinivasan B, Tan DT, Tandon R, Tseng SC, Ueta M, Chodosh J, 2016b. Stevens-johnson syndrome/toxic epidermal necrolysis–A comprehensive review and guide to therapy. I. Systemic disease. Ocul. Surf 14, 2–19. [DOI] [PubMed] [Google Scholar]
- Kollias AN, Spitzlberger GM, Thurau S, Gruterich M, Lackerbauer CA, 2007. Phototherapeutic keratectomy in children. J. Refract. Surg 23, 703–708. [DOI] [PubMed] [Google Scholar]
- Krachmer JH, Purcell JJ Jr., Young CW, Bucher KD, 1978. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol 96, 2036–2039. [DOI] [PubMed] [Google Scholar]
- Kumar A, Yun H, Funderburgh ML, Du Y, 2021. Regenerative therapy for the cornea. Prog. Retin. Eye Res 101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NR, Khamar P, Shetty R, Sharma A, Shetty N, Pahuja N, Abilash VG, Jhanji V, Ghosh A, Mohan RR, Vangala RK, Ghosh A, 2019. Identification of novel predictive factors for post surgical corneal haze. Sci. Rep 9, 16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu G, D’Souza S, Lalgudi VG, Arora V, Chhabra A, Deshpande K, Shetty R, 2020. Photorefractive keratectomy (PRK) Prediction, Examination, tReatment, Follow-up, Evaluation, Chronic Treatment (PERFECT) protocol - a new algorithmic approach for managing post PRK haze. Indian J. Ophthalmol 68, 2950–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali N, 2020. Corneal stromal regeneration: current status and future therapeutic potential. Curr. Eye Res 45, 278–290. [DOI] [PubMed] [Google Scholar]
- Lee GR, 2018. The balance of Th17 versus treg cells in autoimmunity. Int. J. Mol. Sci 19, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Tseng SC, 1995. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J. Cell. Physiol 163, 61–79. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW, 2011. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. Faseb. J 25, 2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Burns AR, Rumbaut RE, Smith CW, 2007. gamma delta T cells are necessary for platelet and neutrophil accumulation in limbal vessels and efficient epithelial repair after corneal abrasion. Am. J. Pathol 171, 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner AL, Chan T, 2021. Precision regenerative medicine. Stem Cell Res. Ther 12, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RR, Tan A, Liu YC, Barathi VA, Mohan RR, Mehta JS, Chaurasia SS, 2016. ITF2357 transactivates Id3 and regulate TGFbeta/BMP7 signaling pathways to attenuate corneal fibrosis. Sci. Rep 6, 20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch W, Weiss JS, 2020. Early and late clinical landmarks of corneal dystrophies. Exp. Eye Res 198, 108139. [DOI] [PubMed] [Google Scholar]
- Liu CY, Arteaga AC, Fung SE, Cortina MS, Leyngold IM, Aakalu VK, 2021. Corneal neurotization for neurotrophic keratopathy: review of surgical techniques and outcomes. Ocul. Surf 20, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li Z, 2021. Resident innate immune cells in the cornea. Front. Immunol 12, 620284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xue Y, Dong D, Xiao C, Lin C, Wang H, Song F, Fu T, Wang Z, Chen J, Pan H, Li Y, Cai D, Li Z, 2017. CCR2(−) and CCR2(+) corneal macrophages exhibit distinct characteristics and balance inflammatory responses after epithelial abrasion. Mucosal Immunol. 10, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Smith CW, Zhang W, Burns AR, Li Z, 2012. NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing. Am. J. Pathol 181, 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Wu RR, Ninomiya Y, Sado Y, Maguen E, Nesburn AB, Kenney MC, 1996. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest. Ophthalmol. Vis. Sci 37, 997–1007. [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M, 2015. Progress in corneal wound healing. Prog. Retin. Eye Res 49, 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo-Gimeno F, Tan DT, Mehta JS, 2011. Evolution of deep anterior lamellar keratoplasty (DALK). Ocul. Surf 9, 98–110. [DOI] [PubMed] [Google Scholar]
- Maharajan N, Cho GW, Choi JH, Jang CH, 2021. Regenerative therapy using umbilical cord serum. In Vivo 35, 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana PK, Sharma N, Vajpayee RB, 2013. Acute corneal hydrops in keratoconus. Indian J. Ophthalmol 61, 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafa MM, Coleman JE, Bowman RW, Rohrich RJ, 2016. Perioperative corneal abrasion: updated guidelines for prevention and management. Plast. Reconstr. Surg 137, 790e–798e. [DOI] [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK, 2001. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest. Ophthalmol. Vis. Sci 42, 2490–2495. [PubMed] [Google Scholar]
- Manzur Yarur F, Ordenes G, Cruzat A, 2021. Autologous serum compared to artificial tear drops for dry eye disease. Medwave 21, e8213. [DOI] [PubMed] [Google Scholar]
- Marlo TL, Giuliano EA, Tripathi R, Sharma A, Mohan RR, 2018. Altering equine corneal fibroblast differentiation through Smad gene transfer. Vet. Ophthalmol 21, 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LM, Jeyabalan N, Tripathi R, Panigrahi T, Johnson PJ, Ghosh A, Mohan RR, 2019. Autophagy in corneal health and disease: a concise review. Ocul. Surf 17, 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, 1998. TGF-beta signal transduction. Annu. Rev. Biochem 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Matthyssen S, Van den Bogerd B, Dhubhghaill SN, Koppen C, Zakaria N, 2018. Corneal regeneration: a review of stromal replacements. Acta Biomater. 69, 31–41. [DOI] [PubMed] [Google Scholar]
- McKay TB, Priyadarsini S, Karamichos D, 2019. Mechanisms of collagen crosslinking in diabetes and keratoconus. Cells 8, 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TB, Priyadarsini S, Karamichos D, 2022. Sex hormones, growth hormone, and the cornea. Cells 11, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt PM, Mohan RR, 2020. The need for improved therapeutic approaches to protect the cornea against chemotoxic injuries. Transl. Vis. Sci. Technol 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CS, Santhiago MR, 2020. Corneal nerves anatomy, function, injury and regeneration. Exp. Eye Res 200, 108243. [DOI] [PubMed] [Google Scholar]
- Meek KM, 2009. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys Rev 1, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek KM, Knupp C, 2015. Corneal structure and transparency. Prog. Retin. Eye Res 49, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menda SA, Das M, Panigrahi A, Prajna NV, Acharya NR, Lietman TM, McLeod SD, Keenan JD, 2020. Association of postfungal keratitis corneal scar features with visual acuity. JAMA Ophthalmol. 138, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelacci YM, 2003. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res 36, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Mijanovic O, Pylaev T, Nikitkina A, Artyukhova M, Brankovic A, Peshkova M, Bikmulina P, Turk B, Bolevich S, Avetisov S, Timashev P, 2021. Tissue engineering meets nanotechnology: molecular mechanism modulations in cornea regeneration. Micromachines 12, 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, Hasan SA, Simmons NL, Stewart MW, 2019. Recurrent corneal erosion: a comprehensive review. Clin. Ophthalmol 13, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotti G, Parodi PC, Zeppieri M, 2021. Stem cell therapy in ocular pathologies in the past 20 years. World J. Stem Cell 13, 366–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal SK, Foulsham W, Shukla S, Elbasiony E, Omoto M, Chauhan SK, 2019. Mesenchymal stromal cells modulate corneal alloimmunity via secretion of hepatocyte growth factor. Stem Cells Transl. Med 8, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadera K, Conatser L, Llanga TA, Carlin K, O’Donnell P, Bagel J, Song L, Kurtzberg J, Samulski RJ, Gilger B, Hirsch ML, 2020. Intrastromal gene therapy prevents and reverses advanced corneal clouding in a canine model of Mucopolysaccharidosis I. Mol. Ther 28, 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobaraki M, Abbasi R, Omidian Vandchali S, Ghaffari M, Moztarzadeh F, Mozafari M, 2019. Corneal repair and regeneration: current concepts and future directions. Front. Bioeng. Biotechnol 7, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Balne PK, Muayad MS, Tripathi R, Sinha NR, Gupta S, An JA, Sinha PR, Hesemann NP, 2021a. Six-month in vivo safety profiling of topical ocular AVV5-decorin gene transfer. Transl. Vis. Sci. Technol 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S, 2010. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp. Eye Res 91, 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R Jr., Zieske JD, Wilson SE, 2003a. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res 76, 71–87. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Martin LM, Sinha NR, 2021b. Novel insights into gene therapy in the cornea. Exp. Eye Res 202, 108361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Mohan RR, Kim WJ, Wilson SE, 2000. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest. Ophthalmol. Vis. Sci 41, 1327–1336. [PubMed] [Google Scholar]
- Mohan RR, Rodier JT, Sharma A, 2013. Corneal gene therapy: basic science and translational perspective. Ocul. Surf 11, 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Schultz GS, Hong JW, Mohan RR, Wilson SE, 2003b. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp. Eye Res 76, 373–383. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE, 2005. Gene therapy in the cornea. Prog. Retin. Eye Res 24, 537–559. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Sinha S, Tandon A, Gupta R, Tovey JC, Sharma A, 2011a. Efficacious and safe tissue-selective controlled gene therapy approaches for the cornea. PLoS One 6, e18771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC, 2011b. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Invest. Ophthalmol. Vis. Sci 52, 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A, 2011c. Decorin biology, expression, function and therapy in the cornea. Curr. Mol. Med 11, 110–128. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Sharma A, Schultz GS, Cowden JW, Tandon A, 2011d. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One 6, e26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Sharma A, Tandon A, 2012. Gene therapy in the cornea: 2005–present. Prog. Retin. Eye Res 31, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Wilson SE, 1999. Ex vivo human corneal epithelial cells express membrane-bound precursor and mature soluble epidermal growth factor (EGF) and transforming growth factor (TGF) alpha proteins. Exp. Eye Res 68, 129–131. [DOI] [PubMed] [Google Scholar]
- Mounsey AL, Gray RE, 2016. Topical antihistamines and mast cell stabilizers for treating allergic conjunctivitis. Am. Fam. Physician 93, 915–916. [PubMed] [Google Scholar]
- Mun Y, Hwang JS, Shin YJ, 2021. Role of neutrophils on the ocular surface. Int. J. Mol. Sci 22, 10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murueta-Goyena A, Canadas P, 2018. Visual outcomes and management after corneal refractive surgery: a review. J. Opt 11, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoji KN, Sun M, Elliott G, Moreno IY, Hughes C, Gesteira TF, Coulson- Thomas VJ, 2021. Extracellular matrix deposition and remodeling after corneal alkali burn in mice. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair AP, D’Souza S, Shetty R, Ahuja P, Kundu G, Khamar P, Dadachanji Z, Paritekar P, Patel P, Dickman MM, Nuijts RM, Mohan RR, Ghosh A, Sethu S, 2021. Altered ocular surface immune cell profile in patients with dry eye disease. Ocul. Surf 21, 96–106. [DOI] [PubMed] [Google Scholar]
- Nicholas MP, Mysore N, 2021. Corneal neovascularization. Exp. Eye Res 202, 108363. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY, 1995. Effect of cytokine-induced migration of Langerhans cells on corneal allograft survival. Eye 9 (Pt 2), 215–218. [DOI] [PubMed] [Google Scholar]
- Nishida T, 2010. Commanding roles of keratocytes in health and disease. Cornea 29 (Suppl. 1), S3–S6. [DOI] [PubMed] [Google Scholar]
- Nishtala K, Pahuja N, Shetty R, Nuijts RM, Ghosh A, 2016. Tear biomarkers for keratoconus. Eye Vis (Lond) 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TP, Li Q, Ashraf MF, Matteson DM, Stark WJ, Chan CC, 1998. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch. Ophthalmol 116, 1470–1474. [DOI] [PubMed] [Google Scholar]
- Oie Y, Nishida K, 2013. Regenerative medicine for the cornea. BioMed Res. Int 2013, 428247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahuja N, Kumar NR, Shroff R, Shetty R, Nuijts RM, Ghosh A, Sinha-Roy A, Chaurasia SS, Mohan RR, Ghosh A, 2016. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest. Ophthalmol. Vis. Sci 57, 5372–5382. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Rajaee SM, Sahebkar A, 2017. Ocular effects of sulfur mustard and therapeutic approaches. J. Cell. Biochem 118, 3549–3560. [DOI] [PubMed] [Google Scholar]
- Pasha H, Palazzolo L, Prakash G, Jhanji V, 2021. Update on corneal collagen crosslinking for ectasia. Curr. Opin. Ophthalmol 32, 343–347. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Alshaker H, Babovic R, Maw K, 2021. A Case of Severe Dry Eye Disease with Corneal Melting as Presenting Complaint of Acute Myeloid Leukaemia, vol. 9. SAGE Open Med Case Rep, 2050313X21994411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Rama P, Mavilio F, De Luca M, 2009. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J. Pathol 217, 217–228. [DOI] [PubMed] [Google Scholar]
- Perez VL, 2017. Visualization of immune responses in the cornea. Cornea 36 (Suppl. 1), S5–S8. [DOI] [PubMed] [Google Scholar]
- Price JG, Idoyaga J, Salmon H, Hogstad B, Bigarella CL, Ghaffari S, Leboeuf M, Merad M, 2015. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat. Immunol 16, 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D, 2020. Diabetic keratopathy: insights and challenges. Surv. Ophthalmol 65, 513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz YS, 1998. Keratoconus. Surv Ophthalmol 42, 297–319. [DOI] [PubMed] [Google Scholar]
- Rajan MS, O’Brart D, Jaycock P, Marshall J, 2006. Effects of ablation diameter on long-term refractive stability and corneal transparency after photorefractive keratectomy. Ophthalmology 113, 1798–1806. [DOI] [PubMed] [Google Scholar]
- Rasiah PK, Geier B, Jha KA, Gangaraju R, 2021. Visual deficits after traumatic brain injury. Histol. Histopathol 36, 711–724. [DOI] [PubMed] [Google Scholar]
- Rigas B, Huang W, Honkanen R, 2020. NSAID-induced corneal melt: clinical importance, pathogenesis, and risk mitigation. Surv. Ophthalmol 65, 1–11. [DOI] [PubMed] [Google Scholar]
- Ritter T, Wilk M, Nosov M, 2013. Gene therapy approaches to prevent corneal graft rejection: where do we stand? Ophthalmic Res. 50, 135–140. [DOI] [PubMed] [Google Scholar]
- Rodier JT, Tripathi R, Fink MK, Sharma A, Korampally M, Gangopadhyay S, Giuliano EA, Sinha PR, Mohan RR, 2019. Linear polyethylenimine-DNA nanoconstruct for corneal gene delivery. J. Ocul. Pharmacol. Therapeut 35, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux LN, Petit I, Domart R, Concordet JP, Qu J, Zhou H, Joliot A, Ferrigno O, Aberdam D, 2018. Modeling of aniridia-related keratopathy by CRISPR/Cas9 genome editing of human limbal epithelial cells and rescue by recombinant PAX6 protein. Stem Cell. 36, 1421–1429. [DOI] [PubMed] [Google Scholar]
- Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK, 2018. Mast cells initiate the recruitment of neutrophils following ocular surface injury. Invest. Ophthalmol. Vis. Sci 59, 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhiago MR, Singh V, Barbosa FL, Agrawal V, Wilson SE, 2011. Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp. Eye Res 93, 786–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS, 2022. Keratoconus: an Updated Review. Cont Lens Anterior Eye, p. 101559. [DOI] [PubMed] [Google Scholar]
- Shah R, Amador C, Tormanen K, Ghiam S, Saghizadeh M, Arumugaswami V, Kumar A, Kramerov AA, Ljubimov AV, 2021. Systemic diseases and the cornea. Exp. Eye Res 204, 108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag SS, Basu S, 2021. Commentary: the role of amniotic membrane transplantation in the management of acute ocular chemical burns. Indian J. Ophthalmol 69, 64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag SS, Chanda S, Donthineni PR, Sane SS, Priyadarshini SR, Basu S, 2020. Clinical clues predictive of Stevens-Johnson syndrome as the cause of chronic cicatrising conjunctivitis. Br. J. Ophthalmol 104, 1005–1009. [DOI] [PubMed] [Google Scholar]
- Shanbhag SS, Patel CN, Goyal R, Donthineni PR, Singh V, Basu S, 2019. Simple limbal epithelial transplantation (SLET): review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J. Ophthalmol 67, 1265–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Bak-Nielsen S, Hjortdal J, Karamichos D, 2018. Pathogenesis of Keratoconus: the intriguing therapeutic potential of Prolactin-inducible protein. Prog. Retin. Eye Res 67, 150–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Anumanthan G, Reyes M, Chen H, Brubaker JW, Siddiqui S, Gupta S, Rieger FG, Mohan RR, 2016. Epigenetic modification prevents excessive wound healing and scar formation after glaucoma filtration surgery. Invest. Ophthalmol. Vis. Sci 57, 3381–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Ghosh A, Hansen ET, Newman JM, Mohan RR, 2010a. Transduction efficiency of AAV 2/6, 2/8 and 2/9 vectors for delivering genes in human corneal fibroblasts. Brain Res. Bull 81, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Ghosh A, Siddappa C, Mohan R, 2010b. Gene Therapy for the Cornea. Encyclopedia of the Eye. Academic press, Oxford, pp. 184–194. [Google Scholar]
- Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR, 2009. Trichostatin a inhibits corneal haze in vitro and in vivo. Invest. Ophthalmol. Vis. Sci 50, 2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Rodier JT, Tandon A, Klibanov AM, Mohan RR, 2012. Attenuation of corneal myofibroblast development through nanoparticle-mediated soluble transforming growth factor-beta type II receptor (sTGFbetaRII) gene transfer. Mol. Vis 18, 2598–2607. [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sinha NR, Siddiqui S, Mohan RR, 2015. Role of 5’TG3’-interacting factors (TGIFs) in vorinostat (HDAC inhibitor)-mediated corneal fibrosis inhibition. Mol. Vis 21, 974–984. [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tandon A, Tovey JC, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR, 2011. Polyethylenimine-conjugated gold nanoparticles: gene transfer potential and low toxicity in the cornea. Nanomedicine 7, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Soni D, Mohan RR, Sarkar D, Gupta R, Chauhan K, Karkhur S, Morya AK, 2021. Corticosteroids in the management of infectious keratitis: a concise review. J. Ocul. Pharmacol. Therapeut 37, 452–463. [DOI] [PubMed] [Google Scholar]
- Sharma N, Kaur M, Agarwal T, Sangwan VS, Vajpayee RB, 2018. Treatment of acute ocular chemical burns. Surv. Ophthalmol 63, 214–235. [DOI] [PubMed] [Google Scholar]
- Shaughnessy MP, Ellis FJ, Jeffery AR, Szczotka L, 2001. Rigid gas-permeable contact lenses are a safe and effective means of treating refractive abnormalities in the pediatric population. CLAO J. 27, 195–201. [PubMed] [Google Scholar]
- Shetty R, D’Souza S, Khamar P, Ghosh A, Nuijts R, Sethu S, 2020. Biochemical markers and alterations in keratoconus. Asia Pac. J. Ophthalmol. (Phila) 9, 533–540. [DOI] [PubMed] [Google Scholar]
- Shetty R, Kaweri L, Pahuja N, Nagaraja H, Wadia K, Jayadev C, Nuijts R, Arora V, 2015. Current review and a simplified “five-point management algorithm” for keratoconus. Indian J. Ophthalmol 63, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty R, Kumar NR, Subramani M, Krishna L, Murugeswari P, Matalia H, Khamar P, Dadachanji ZV, Mohan RR, Ghosh A, Das D, 2021. Safety and efficacy of combination of suberoylamilide hydroxyamic acid and mitomycin C in reducing pro-fibrotic changes in human corneal epithelial cells. Sci. Rep 11, 4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzaei Sani E, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A, Khademhosseini A, Dana R, Annabi N, 2019. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv 5, eaav1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Shanbhag SS, Tavakkoli F, Varma S, Singh V, Basu S, 2020. Limbal epithelial and mesenchymal stem cell therapy for corneal regeneration. Curr. Eye Res 45, 265–277. [DOI] [PubMed] [Google Scholar]
- Sinha NR, Balne PK, Bunyak F, Hofmann AC, Lim RR, Mohan RR, Chaurasia SS, 2021. Collagen matrix perturbations in corneal stroma of Ossabaw mini pigs with type 2 diabetes. Mol. Vis 27, 666–678. [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Naderan M, 2020. Management strategies of ocular chemical burns: current perspectives. Clin. Ophthalmol 14, 2687–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton WM, Chaurasia SS, Medeiros FW, Mohan RR, Sinha S, Wilson SE, 2008. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res 86, 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Sun S, Tian B, Tai PWL, Luo Y, Ko J, Zhan W, Ke X, Zheng Q, Li X, Yan H, Gao G, Lin H, 2021. Efficacious, safe, and stable inhibition of corneal neovascularization by AAV-vectored anti-VEGF therapeutics. Mol. Ther Methods Clin. Dev 22, 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka T, Iwanishi H, Okada Y, Miyajima M, Ichikawa K, Reinach PS, Matsumoto KI, Saika S, 2021. Impairment of corneal epithelial wound healing is association with increased neutrophil infiltration and reactive oxygen species activation in tenascin X-deficient mice. Lab. Invest 101, 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zafrullah N, Adams S, Devaux F, Avila MY, Ziebarth N, Margo CE, Koch M, Espana EM, 2021. Collagen XIV is an intrinsic regulator of corneal stromal structure and function. Am. J. Pathol 191, 2184–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zafrullah N, Devaux F, Hemmavanh C, Adams S, Ziebarth NM, Koch M, Birk DE, Espana EM, 2020. Collagen XII is a regulator of corneal stroma structure and function. Invest. Ophthalmol. Vis. Sci 61, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DT, Ang LP, 2004. Automated lamellar therapeutic keratoplasty for post-PRK corneal scarring and thinning. Am. J. Ophthalmol 138, 1067–1069. [DOI] [PubMed] [Google Scholar]
- Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR, 2013. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS One 8, e66434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR, 2010. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med 10, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Waggoner MR, Sharma A, Cowden JW, Gibson DJ, Liu Y, Schultz GS, Mohan RR, 2012. Vorinostat: a potent agent to prevent and treat laser-induced corneal haze. J. Refract. Surg 28, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thylefors B, 1992. Epidemiological patterns of ocular trauma. Aust. N. Z. J. Ophthalmol 20, 95–98. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE, 2016. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res 142, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE, 2013. The corneal epithelial basement membrane: structure, function, and disease. Invest. Ophthalmol. Vis. Sci 54, 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R, Balne PK, Sinha NR, Martin LM, Kamil S, Landreneau JR, Gupta S, Rodier JT, Sinha PR, Hesemann NP, Hofmann AC, Fink MK, Chaurasia SS, Mohan RR, 2020. A novel topical ophthalmic formulation to mitigate acute mustard gas keratopathy in vivo: a pilot study. Transl. Vis. Sci. Technol 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuft SJ, Gregory WM, Buckley RJ, 1994. Acute corneal hydrops in keratoconus. Ophthalmology 101, 1738–1744. [DOI] [PubMed] [Google Scholar]
- Ucgul RK, Celebi S, Yilmaz NS, Bukan N, Ucgul AY, 2021. Intrastromal versus subconjunctival anti-VEGF agents for treatment of corneal neovascularization: a rabbit study. Eye 35, 3123–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Essen TH, Lin CC, Hussain AK, Maas S, Lai HJ, Linnartz H, van den Berg TJ, Salvatori DC, Luyten GP, Jager MJ, 2013. A fish scale-derived collagen matrix as artificial cornea in rats: properties and potential. Invest. Ophthalmol. Vis. Sci 54, 3224–3233. [DOI] [PubMed] [Google Scholar]
- Vasquez-Perez A, Zarei-Ghanavati M, Avadhanam V, Liu C, 2018. Osteo-odonto-keratoprosthesis in severe thermal and chemical injuries. Cornea 37, 993–999. [DOI] [PubMed] [Google Scholar]
- Vedana G, Villarreal G Jr., Jun AS, 2016. Fuchs endothelial corneal dystrophy: current perspectives. Clin. Ophthalmol 10, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard AH, 2014. Past and present of corneal refractive surgery: a retrospective study of long-term results after photorefractive keratectomy and a prospective study of refractive lenticule extraction. Acta Ophthalmol. 92 Thesis 2, 1–21. [DOI] [PubMed] [Google Scholar]
- Vohra V, Shetty R, James E, Kundu G, D’Souza S, 2021a. Evaluating the safety and efficacy of compression sutures with intracameral perfluoropropane (C3F8) in the management of acute corneal hydrops. Int. Ophthalmol 41, 2027–2031. [DOI] [PubMed] [Google Scholar]
- Vohra V, Tuteja S, Chawla H, 2021b. Collagen Cross Linking for Keratoconus, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Wang F, Ma F, Song Y, Li N, Li X, Pang Y, Hu P, Shao A, Deng C, Zhang X, 2020. Topical administration of rapamycin promotes retinal ganglion cell survival and reduces intraocular pressure in a rat glaucoma model. Eur. J. Pharmacol 884, 173369. [DOI] [PubMed] [Google Scholar]
- Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J Jr., Dalton N, Jones Y, Reed CC, Iozzo RV, McCulloch AD, 2005. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 24, 313–324. [DOI] [PubMed] [Google Scholar]
- Whitcher JP, Srinivasan M, 1997. Corneal ulceration in the developing world–a silent epidemic. Br. J. Ophthalmol 81, 622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcher JP, Srinivasan M, Upadhyay MP, 2001. Corneal blindness: a global perspective. Bull. World Health Organ 79, 214–221. [PMC free article] [PubMed] [Google Scholar]
- Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K, 2011. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020a. Bowman’s layer in the cornea- structure and function and regeneration. Exp. Eye Res 195, 108033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020b. Corneal myofibroblasts and fibrosis. Exp. Eye Res 201, 108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, 2020c. Corneal wound healing. Exp. Eye Res 197, 108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SL, El Haj AJ, Yang Y, 2012. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J. Funct. Biomater 3, 642–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lim DK, Lim BXH, Wong N, Hafezi F, Manotosh R, Lim CHL, 2021. Corneal cross-linking: the evolution of treatment for corneal diseases. Front. Pharmacol 12, 686630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang H, Liu S, Chen G, He S, Li Z, Wang L, 2018. Mast cell activation protects cornea by promoting neutrophil infiltration via stimulating ICAM-1 and vascular dilation in fungal keratitis. Sci. Rep 8, 8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam GHF, Riau AK, Funderburgh ML, Mehta JS, Jhanji V, 2020. Keratocyte biology. Exp. Eye Res 196, 108062. [DOI] [PubMed] [Google Scholar]
- Yu FX, Lee PSY, Yang L, Gao N, Zhang Y, Ljubimov AV, Yang E, Zhou Q, Xie L, 2022. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog. Retin. Eye Res 101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Yee MB, Lathrop KL, Kinchington PR, Hendricks RL, St Leger AJ, 2020. Production of the cytokine VEGF-A by CD4(+) T and myeloid cells disrupts the corneal nerve landscape and promotes herpes stromal keratitis. Immunity 53, 1050–1062 e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MC, Liu X, Jin Y, Jiang DL, Wei XS, Xie HT, 2015. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am. J. Transplant 15, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Zheng J, Huang X, Zhang Y, Wang Y, Qin Q, Lin L, Jin X, Lam C, Zhang J, 2019. Short-term results of acellular porcine corneal stroma keratoplasty for herpes simplex keratitis. Xenotransplantation 26, e12509. [DOI] [PubMed] [Google Scholar]
- Zhu XR, Du JH, 2018. Autophagy: a potential target for the treatment of intraocular neovascularization. Int. J. Ophthalmol 11, 695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyablitskaya M, Jayyosi C, Takaoka A, Myers KM, Suh LH, Nagasaki T, Trokel SL, Paik DC, 2020. Topical corneal cross-linking solution delivered via corneal reservoir in Dutch-belted rabbits. Transl. Vis. Sci. Technol 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]


