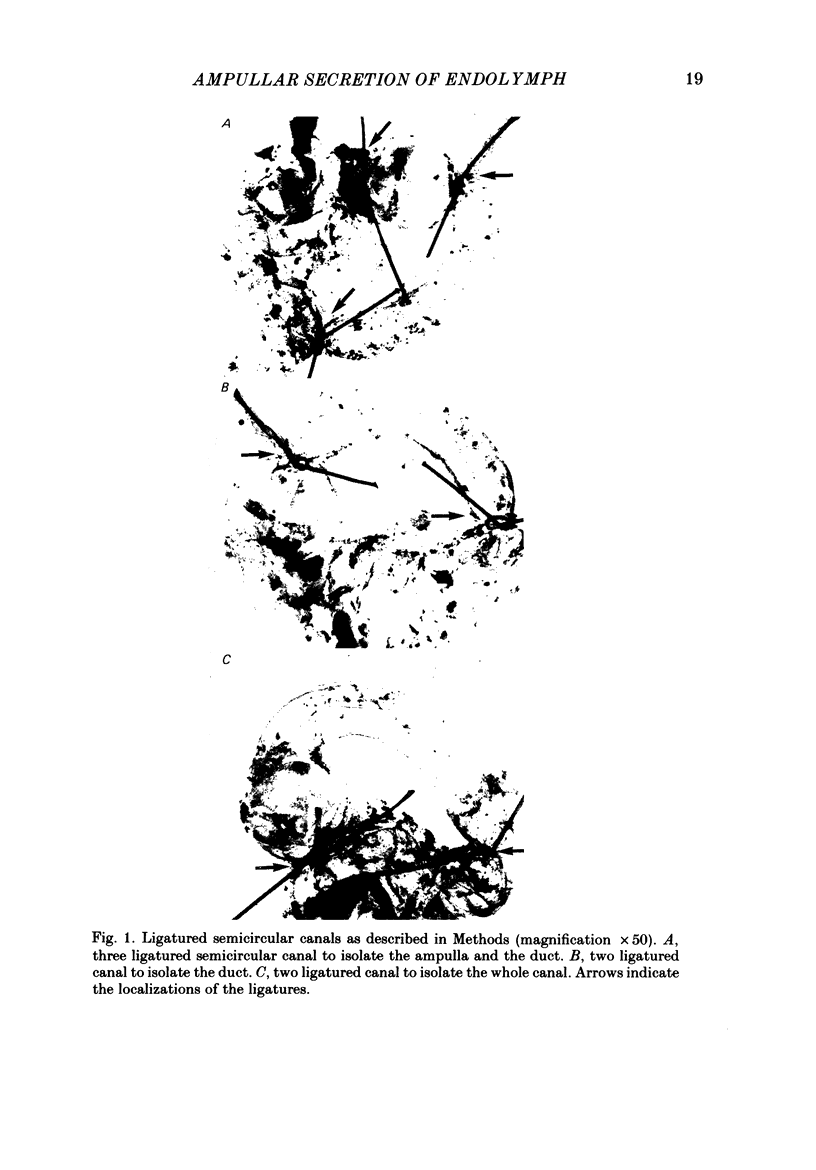
Abstract
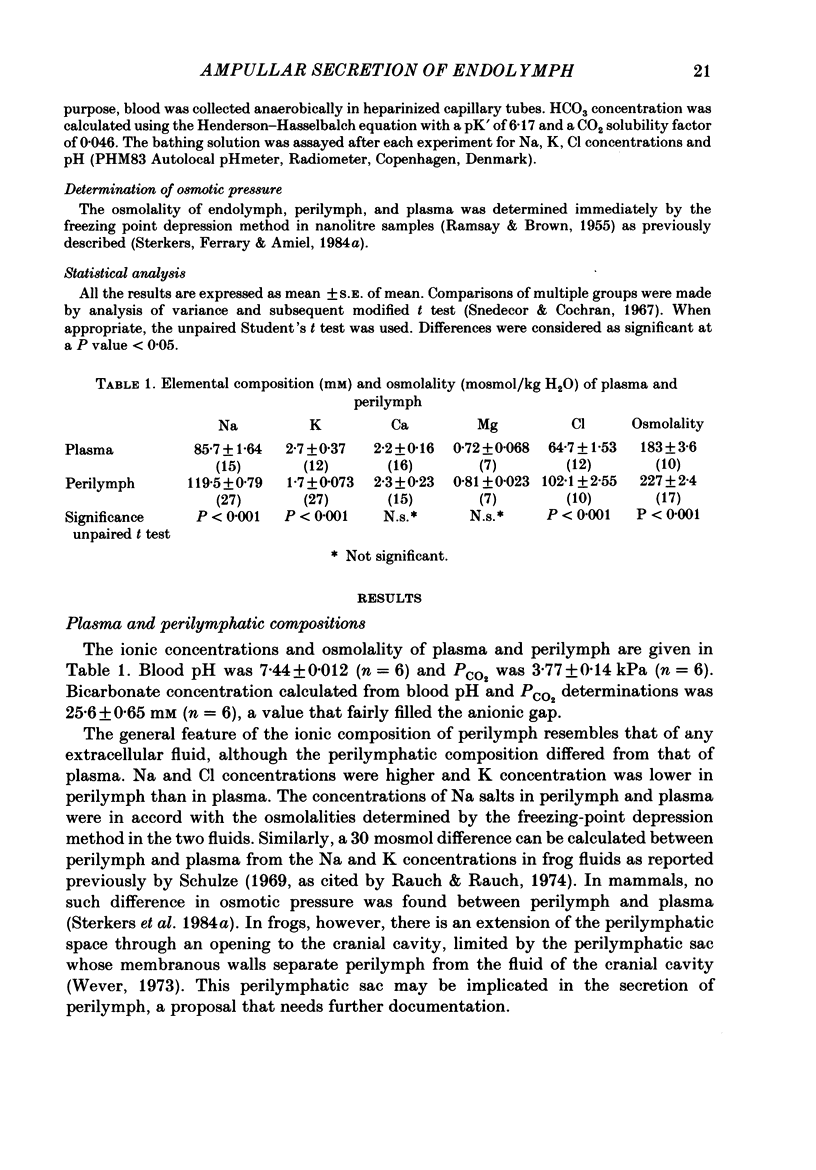
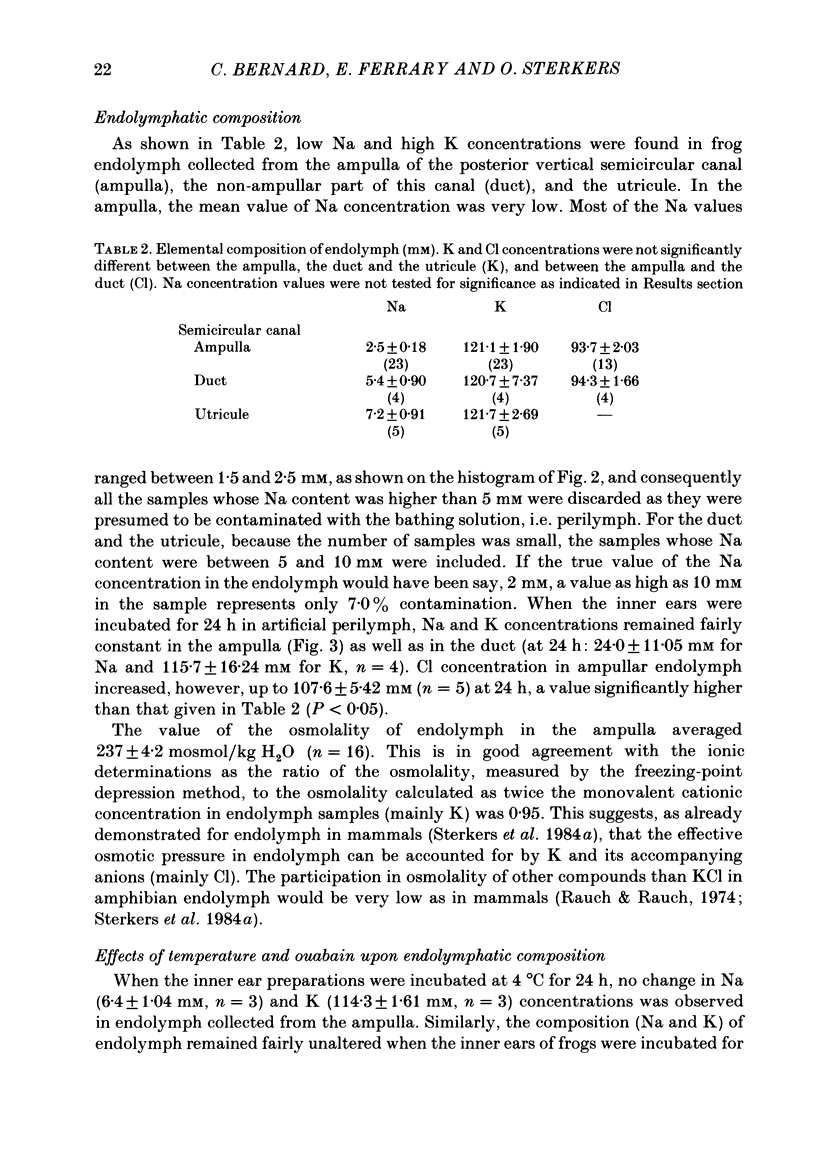
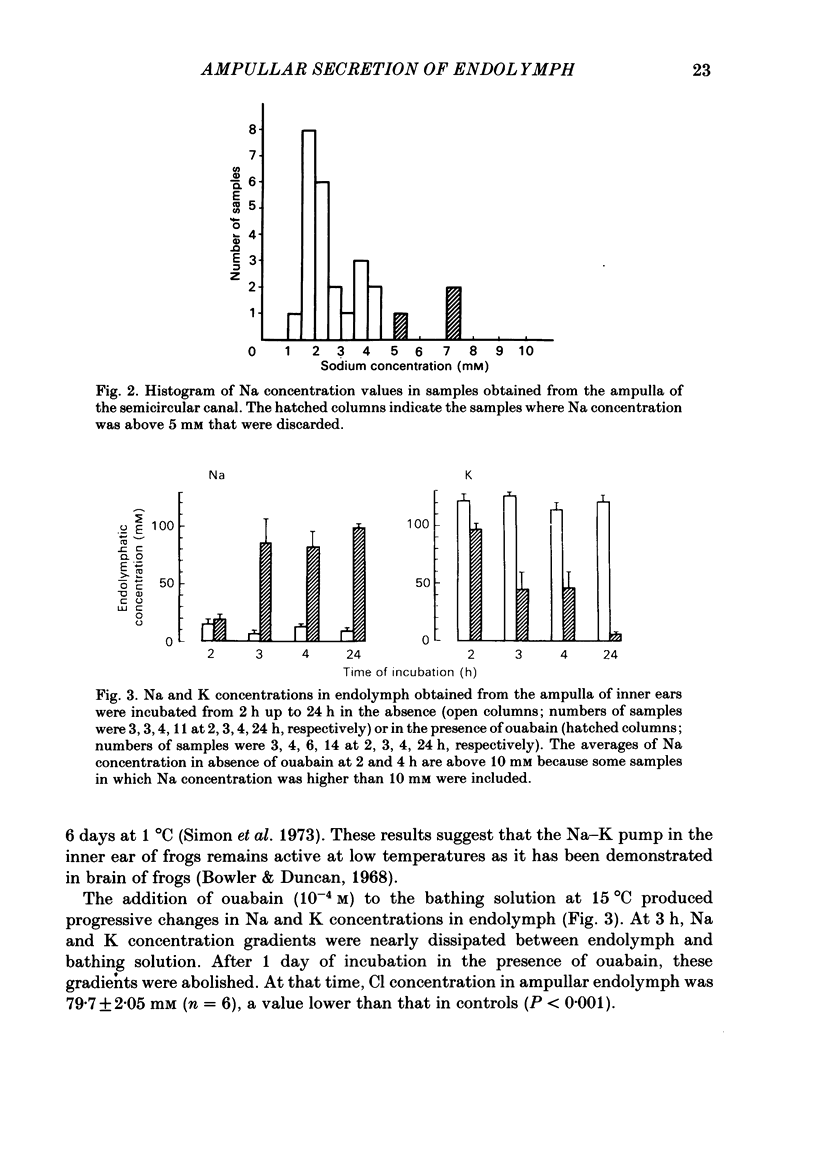
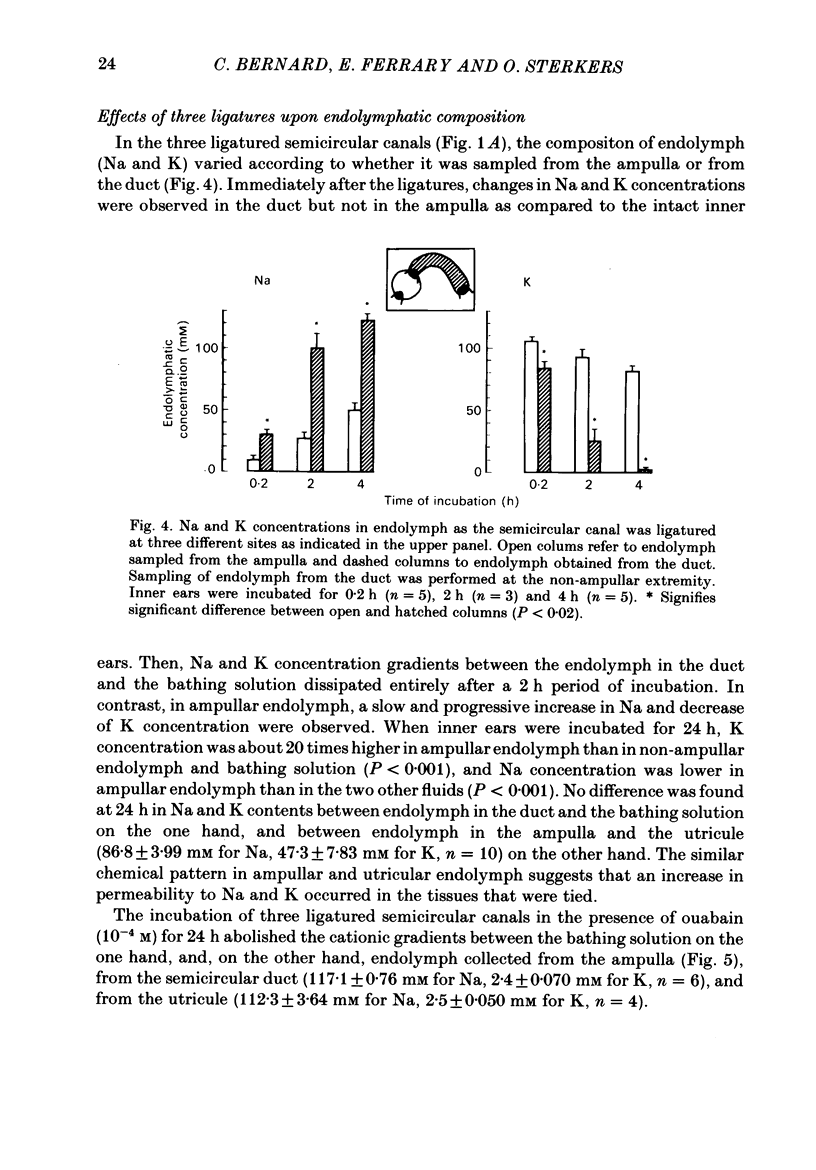
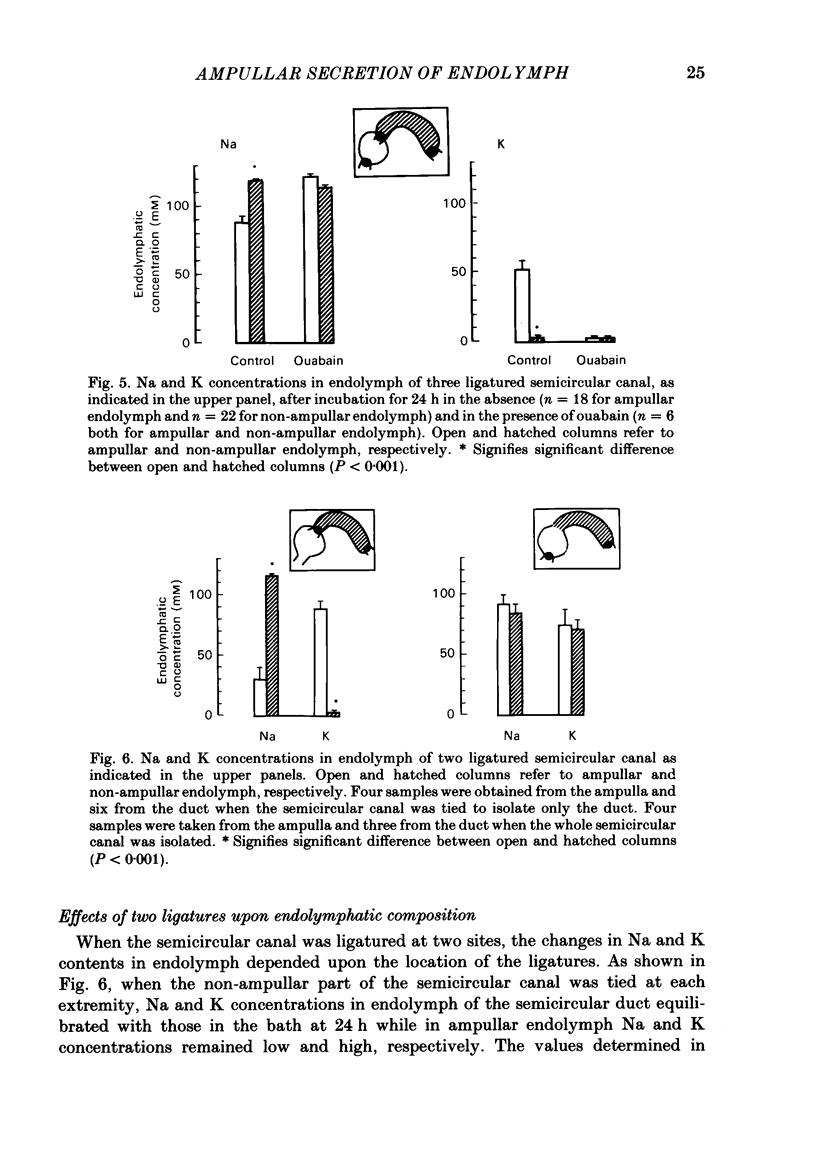
The mechanisms of secretion of endolymph were studied in vitro in the isolated inner ear of the frog. Prior to in vitro experiments, the composition of perilymph was evaluated in vivo and compared to that of plasma. Composition of perilymph resembled that of an extracellular fluid, although Na and Cl concentrations were higher and K concentration was lower in perilymph than in plasma water. No difference in Ca and Mg concentrations was observed between these two fluids. Osmolality averaged 227 mosmol/kg H2O in perilymph and 183 mosmol/kg H2O in plasma. Endolymph in frog inner ear corresponded in chemical pattern to mammalian endolymph. K and Na concentrations in endolymph collected from the ampulla of the posterior vertical semicircular canal averaged 121.1 mM and 2.5 mM, respectively. Osmolality of endolymph was 237 mosmol/kg H2O. K and Na concentrations were unaltered when inner ears were incubated for 24 h either at 15 degrees C or at 4 degrees C. Addition of ouabain (10(-4) M) to the perilymph-like bathing solution altered greatly Na and K composition of endolymph after incubation for 3 h at 15 degrees C. The Na and K concentration gradients between endolymph and the bath were abolished after incubation for 24 h. Ligatures of the posterior vertical semicircular canal were performed at different sites to isolate some parts of the canal, i.e. the ampulla and the non-ampullar duct. K concentration in the ampulla after incubation for 24 h remained as high as 20 times that in the bath. This K gradient was abolished in the presence of ouabain (10(-4) M). High K concentration could be maintained in the non-ampullar part of the semicircular canal only if the latter communicated with the ampulla. It is concluded that endolymph is actively secreted into the ampulla of the semicircular canal. Na+-K+-activated ATPase in the ampullar dark cells may energize the ouabain sensitive ionic transports that are involved in the production of endolymph. Endolymph secreted into the ampulla would spread intraluminally to account for the high K and low Na concentrations of the fluid which fills the non-secretory part of the semicircular canal.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosher S. K. The effects of inhibition of the strial Na+-K+-activated ATPase by perilymphatic ouabain in the guinea pig. Acta Otolaryngol. 1980 Sep-Oct;90(3-4):219–229. doi: 10.3109/00016488009131718. [DOI] [PubMed] [Google Scholar]
- Bosher S. K., Warren R. L. Observations on the electrochemistry of the cochlear endolymph of the rat: a quantitative study of its electrical potential and ionic composition as determined by means of flame spectrophotometry. Proc R Soc Lond B Biol Sci. 1968 Nov 5;171(1023):227–247. doi: 10.1098/rspb.1968.0066. [DOI] [PubMed] [Google Scholar]
- Bowler K., Duncan C. J. The temperature characteristics of the ATPases from a frog brain microsomal preparation. Comp Biochem Physiol. 1968 Jan;24(1):223–227. doi: 10.1016/0010-406x(68)90969-9. [DOI] [PubMed] [Google Scholar]
- Burnham J. A., Stirling C. E. Quantitative localization of Na-K pump site in frog inner ear dark cells. Hear Res. 1984 Mar;13(3):261–268. doi: 10.1016/0378-5955(84)90079-0. [DOI] [PubMed] [Google Scholar]
- Henriksson N. G., Gleisner L., Johansson G. Experimental pressure variations in the membranous labyrinth of the frog. Acta Otolaryngol. 1966 Apr;61(4):281–291. doi: 10.3109/00016486609127065. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE C. G., SCHMIDT R. S., JOHNSTONE B. M. SODIUM AND POTASSIUM IN VERTEBRATE COCHLEAR ENDOLYMPH AS DETERMINED BY FLAME MICROSPECTROPHOTOMETRY. Comp Biochem Physiol. 1963 Aug;9:335–341. doi: 10.1016/0010-406x(63)90008-2. [DOI] [PubMed] [Google Scholar]
- Jahnke K. The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol Suppl. 1975;336:1–40. [PubMed] [Google Scholar]
- Kerr T. P., Ross M. D., Ernst S. A. Cellular localization of Na+,K+-ATPase in the mammalian cochlear duct: significance for cochlear fluid balance. Am J Otolaryngol. 1982 Sep-Oct;3(5):332–338. doi: 10.1016/s0196-0709(82)80006-9. [DOI] [PubMed] [Google Scholar]
- Kimura R. S. Distribution, structure, and function of dark cells in the vestibular labyrinth. Ann Otol Rhinol Laryngol. 1969 Jun;78(3):542–561. doi: 10.1177/000348946907800311. [DOI] [PubMed] [Google Scholar]
- Konishi T., Mendelsohn M. Effect of ouabain on cochlear potentials and endolymph composition in guinea pigs. Acta Otolaryngol. 1970 Mar;69(3):192–199. doi: 10.3109/00016487009123353. [DOI] [PubMed] [Google Scholar]
- Kuijpers W., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XXIV. Localization and properties of ATPase in the inner ear of the guinea pig. Biochim Biophys Acta. 1969 Apr;173(3):477–485. doi: 10.1016/0005-2736(69)90012-1. [DOI] [PubMed] [Google Scholar]
- Kuijpers W., Bonting S. L. The cochlear potentials. I. The effect of ouabain on the cochlear potentials of the guinea pig. Pflugers Arch. 1970;320(4):348–358. doi: 10.1007/BF00588213. [DOI] [PubMed] [Google Scholar]
- Morel F., Lucarain C. Un spectrophotomètre à flamme pour le dosage du sodium et du potassium dans des échantillons biologiques de l'ordre du nanolitre. J Physiol (Paris) 1967;59(4 Suppl):460–461. [PubMed] [Google Scholar]
- Nakai Y., Hilding D. Vestibular endolymph-producing epithelium. Electron microscopic study of the development and histochemistry of the dark cells of the crista ampullaris. Acta Otolaryngol. 1968 Jul-Aug;66(1):120–128. doi: 10.3109/00016486809126280. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R. S., FERNANDEZ C. Labyrinthine DC potentials in representative vertebrates. J Cell Comp Physiol. 1962 Jun;59:311–322. doi: 10.1002/jcp.1030590311. [DOI] [PubMed] [Google Scholar]
- SMITH C. A., LOWRY O. H., WU M. L. The electrolytes of the labyrinthine fluids. Laryngoscope. 1954 Mar;64(3):141–153. doi: 10.1288/00005537-195403000-00001. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Johnstone B. M. Differential effects of ouabain and ethacrynic acid on the labyrinthine potentials. Pflugers Arch. 1974;352(4):339–350. doi: 10.1007/BF00585686. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Johnstone B. M. Production and role of inner ear fluid. Prog Neurobiol. 1975;5(4):337–362. doi: 10.1016/0301-0082(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Hilding D. A., Kashgarian M. Micropuncture study of the mechanism of endolymph production in the frog. Am J Physiol. 1973 Jul;225(1):114–118. doi: 10.1152/ajplegacy.1973.225.1.114. [DOI] [PubMed] [Google Scholar]
- Sterkers O., Ferrary E., Amiel C. Inter- and intracompartmental osmotic gradients within the rat cochlea. Am J Physiol. 1984 Oct;247(4 Pt 2):F602–F606. doi: 10.1152/ajprenal.1984.247.4.F602. [DOI] [PubMed] [Google Scholar]
- Valli P., Zucca G., Casella C. Ionic composition of the endolymph and sensory transduction in labyrinthine organs. Acta Otolaryngol. 1979 May-Jun;87(5-6):466–471. doi: 10.3109/00016487909126453. [DOI] [PubMed] [Google Scholar]
- Wever E. G. The ear and hearing in the frog, Rana pipiens. J Morphol. 1973 Dec;141(4):461–477. doi: 10.1002/jmor.1051410406. [DOI] [PubMed] [Google Scholar]