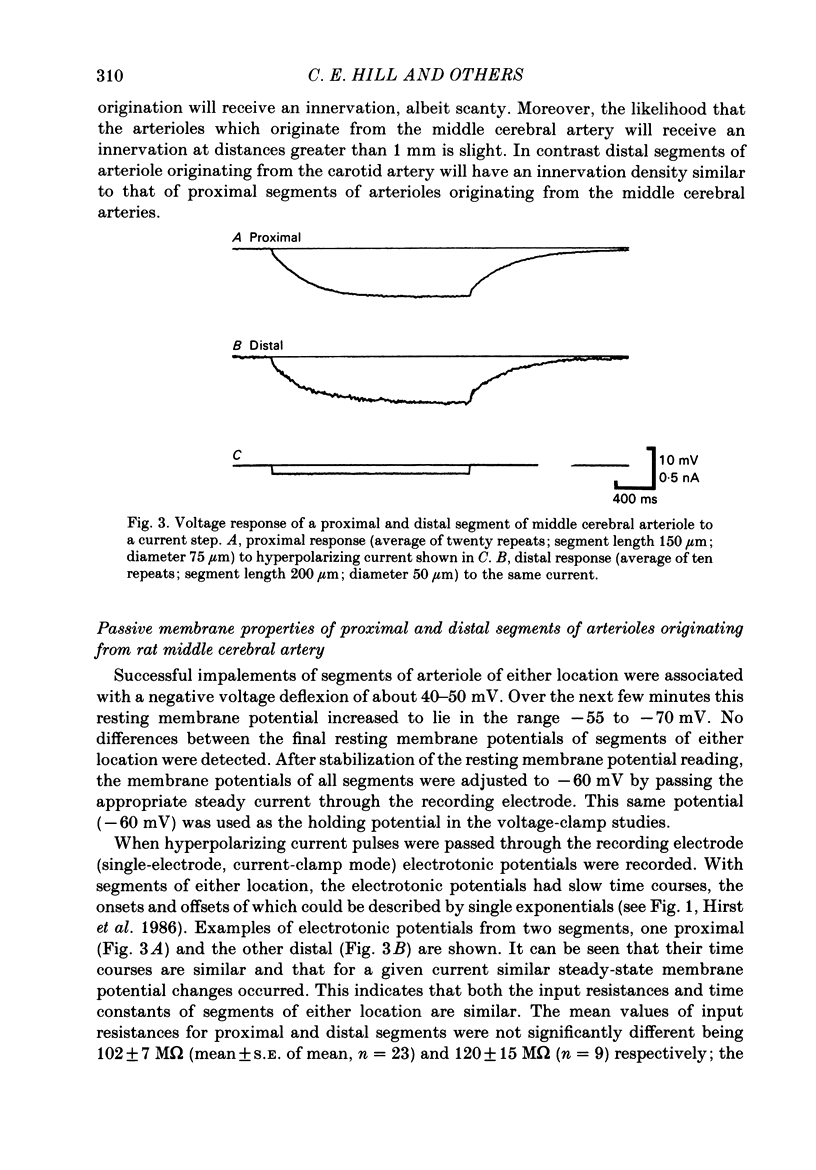
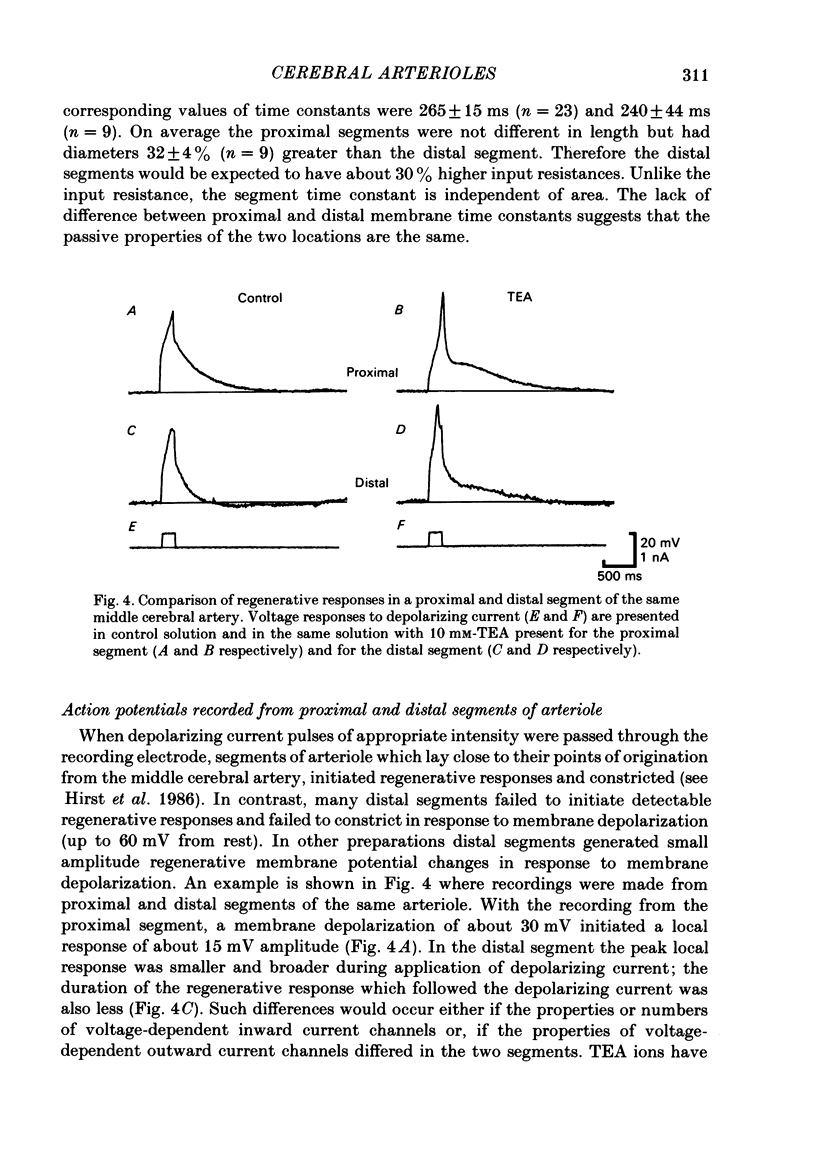
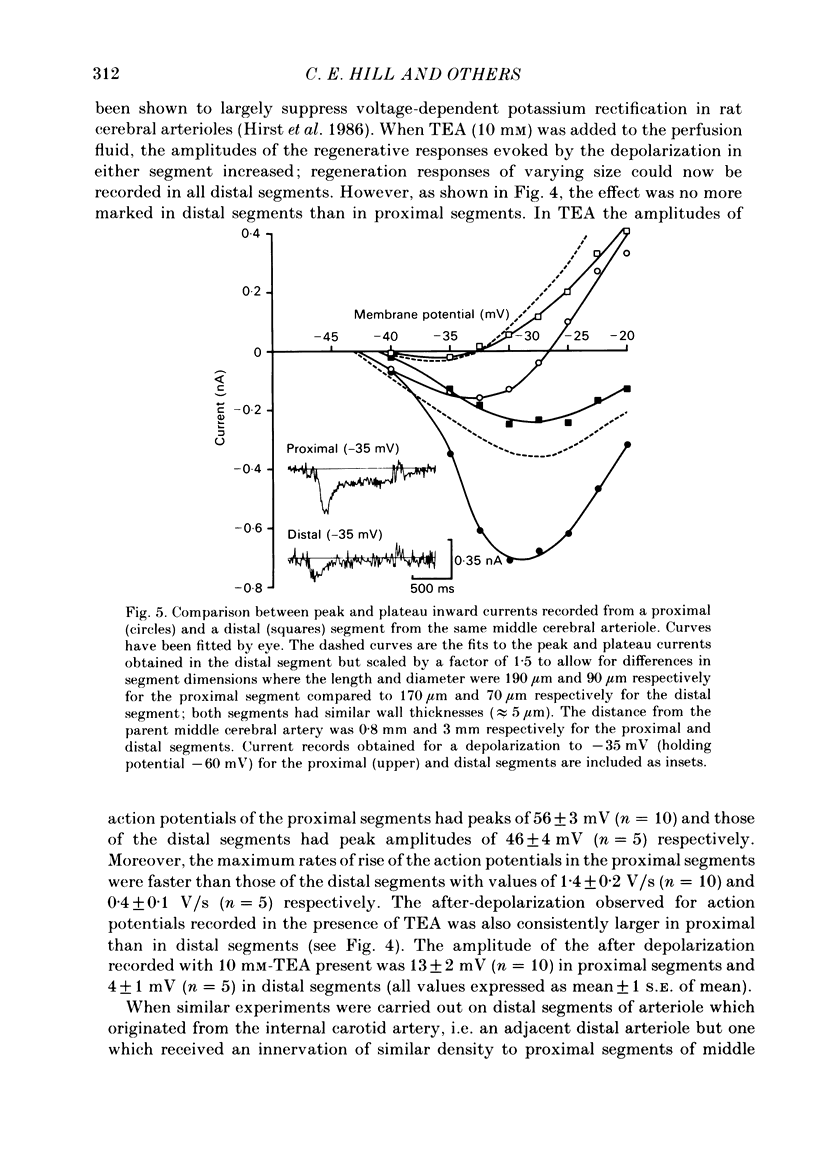
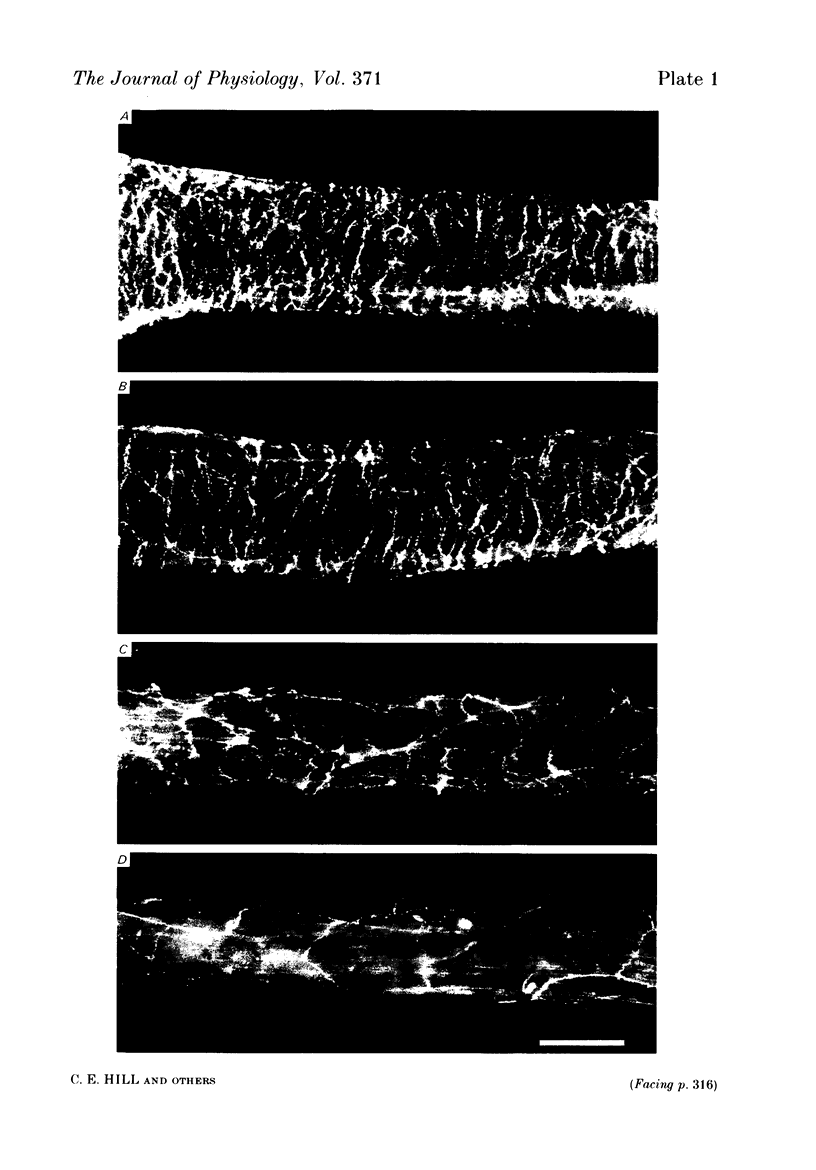
Abstract
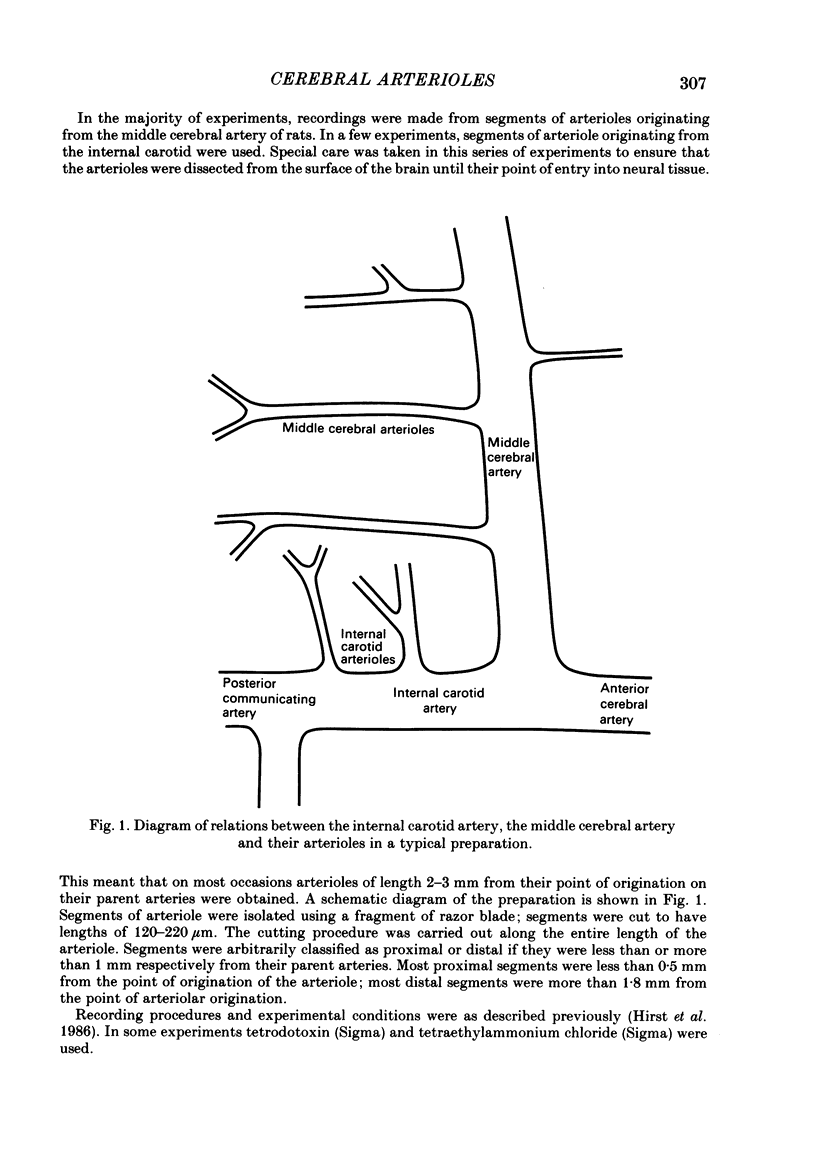
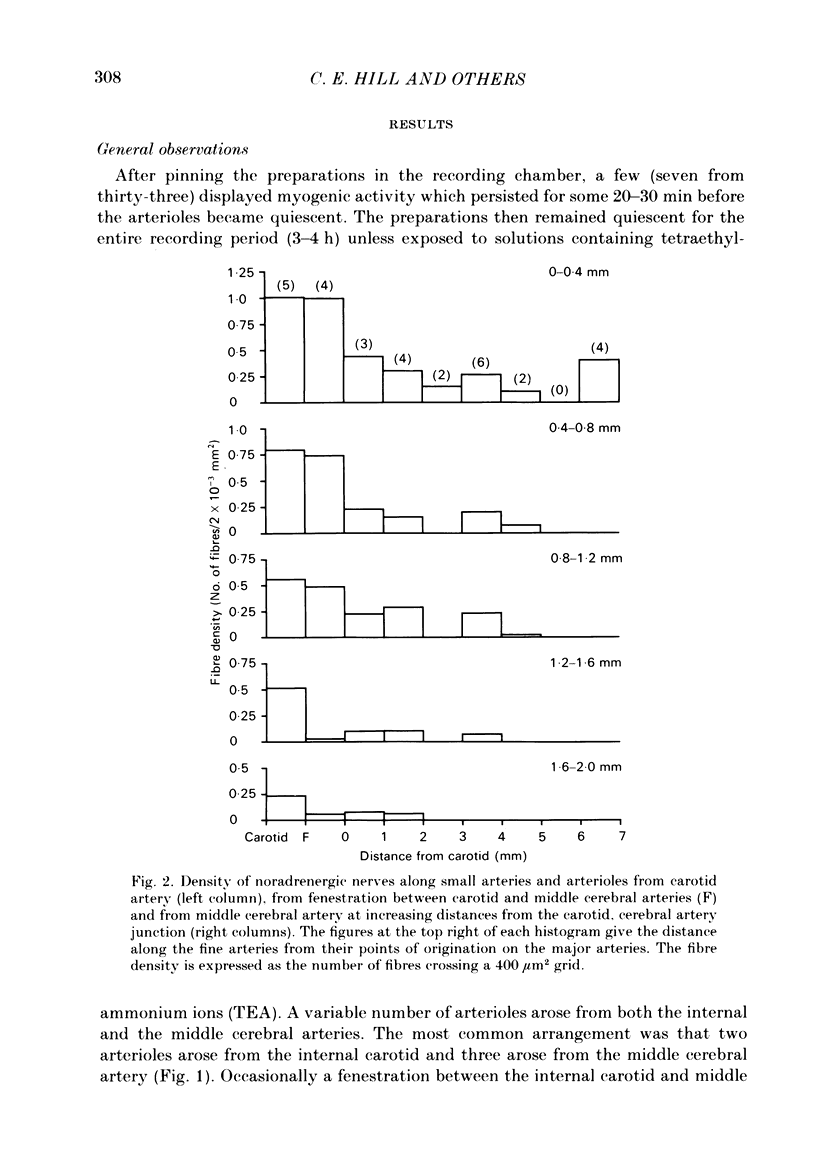
The densities of the adrenergic innervation of the internal carotid and middle cerebral arteries and their extracerebral branches have been determined using fluorescence histochemistry. The density of the nerve plexus on the internal carotid artery was greater than that of the middle cerebral artery. The density of the plexus on the middle cerebral artery decreased with increasing distance from its origin. The density and the peripheral extent of the nerve fibre plexus on the arterioles arising from the carotid artery were greater than those arising from the middle cerebral artery. On any arteriole the density of innervation decreased with increasing distance from its origin. The passive electrical properties of proximal and distal middle cerebral arteriolar segments were compared. Both proximal and distal arteriolar segments had similar resistances and time constants in the order of 100 M omega and 250 ms respectively. Small regenerative responses could be elicited in all proximal middle cerebral arteriolar segments but only in a proportion of corresponding distal segments. The addition of external tetraethylammonium ions (TEA) provided much larger regenerative responses. Action potentials in proximal middle cerebral arteriolar segments had larger peak amplitudes and faster rise times than those of corresponding distal segments. Distal carotid arteriolar segments had similar voltage-dependent excitability as proximal segments of middle cerebral arterioles but generated less inward current for a given voltage step. There was a direct correlation between the density of innervation and the voltage-dependent excitability of arteriolar smooth muscle cells. The possibility that the presence of nerves is correlated with the density of calcium channels is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duckles S. P. Evidence for a functional cholinergic innervation of cerebral arteries. J Pharmacol Exp Ther. 1981 Jun;217(3):544–548. [PubMed] [Google Scholar]
- Falck B., Mchedlishvili G. I., Owman C. Histochemical demonstration of adrenergic nerves in cortex-pia of rabbit. Acta Pharmacol Toxicol (Copenh) 1965;23(2):133–142. doi: 10.1111/j.1600-0773.1965.tb03579.x. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Hirst G. D., Van Helden D. F. Some properties of excitatory junction currents recorded from submucosal arterioles of guinea-pig ileum. J Physiol. 1984 Jun;351:87–98. doi: 10.1113/jphysiol.1984.sp015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence V. M., Bevan J. A. Biochemical determinations of cholinergic innervation in cerebral arteries. Circ Res. 1979 Aug;45(2):212–218. doi: 10.1161/01.res.45.2.212. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Emson P. C., Håkanson R., Moghimzadeh E., Sundler F., Taylor I. L., Chance R. E. Distribution, pathways and reactions to drug treatment of nerves with neuropeptide Y- and pancreatic polypeptide-like immunoreactivity in the guinea-pig digestive tract. Cell Tissue Res. 1983;234(1):71–92. doi: 10.1007/BF00217403. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Wilson A. J. Water-stable fluorophores, produced by reaction with aldehyde solutions, for the histochemical localization of catechol- and indolethylamines. Histochemistry. 1977 May 20;52(2):159–170. doi: 10.1007/BF00492292. [DOI] [PubMed] [Google Scholar]
- Hill C. E., Hirst G. D., van Helden D. F. Development of sympathetic innervation to proximal and distal arteries of the rat mesentery. J Physiol. 1983 May;338:129–147. doi: 10.1113/jphysiol.1983.sp014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silverberg G. D., van Helden D. F. The action potential and underlying ionic currents in proximal rat middle cerebral arterioles. J Physiol. 1986 Feb;371:289–304. doi: 10.1113/jphysiol.1986.sp015975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwayama T., Furness J. B., Burnstock G. Dual adrenergic and cholinergic innervation of the cerebral arteries of the rat. An ultrastructural study. Circ Res. 1970 May;26(5):635–646. doi: 10.1161/01.res.26.5.635. [DOI] [PubMed] [Google Scholar]
- Nielsen K. C., Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res. 1967 Dec;6(4):773–776. doi: 10.1016/0006-8993(67)90134-5. [DOI] [PubMed] [Google Scholar]
- Samarasinghe D. D. The innervation of the cerebral arteries in the rat: an electron microscope study. J Anat. 1965 Oct;99(Pt 4):815–828. [PMC free article] [PubMed] [Google Scholar]
- Sato S. An electron microscopic study on the innervation of the intracranial artery of the rat. Am J Anat. 1966 May;118(3):873–889. doi: 10.1002/aja.1001180312. [DOI] [PubMed] [Google Scholar]