Abstract
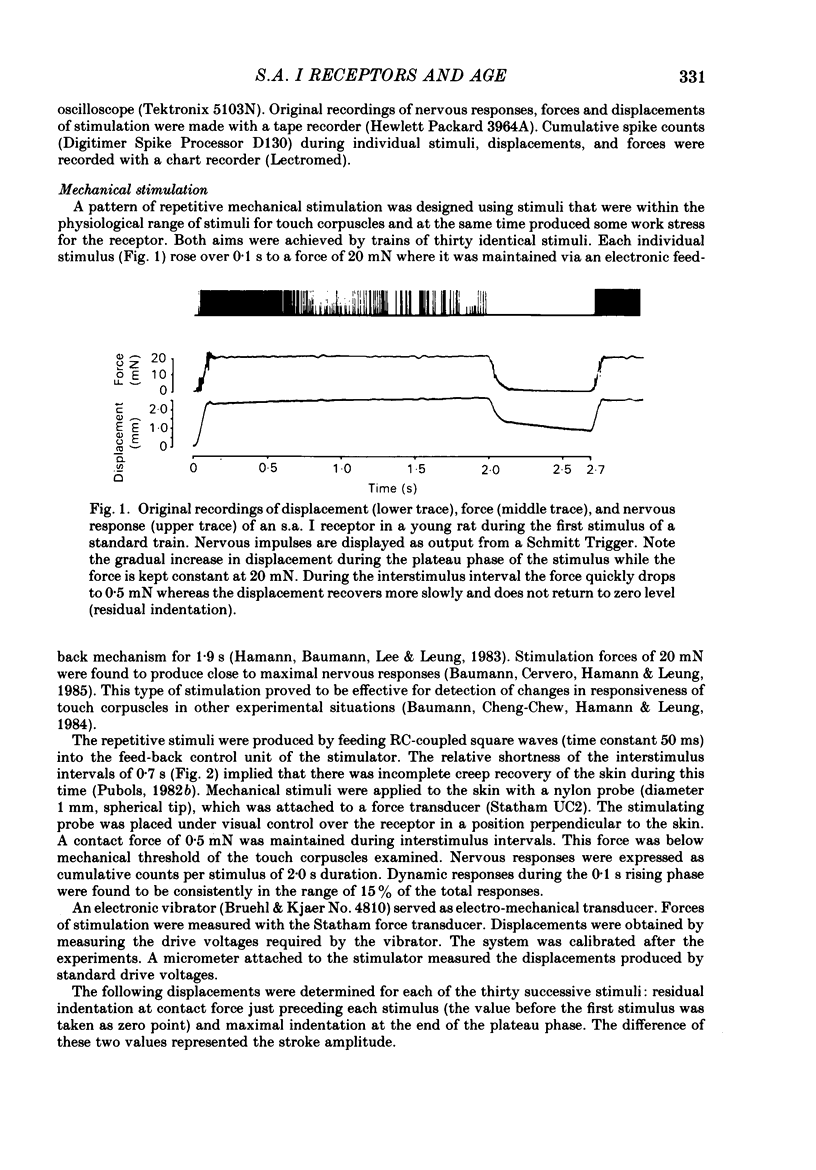
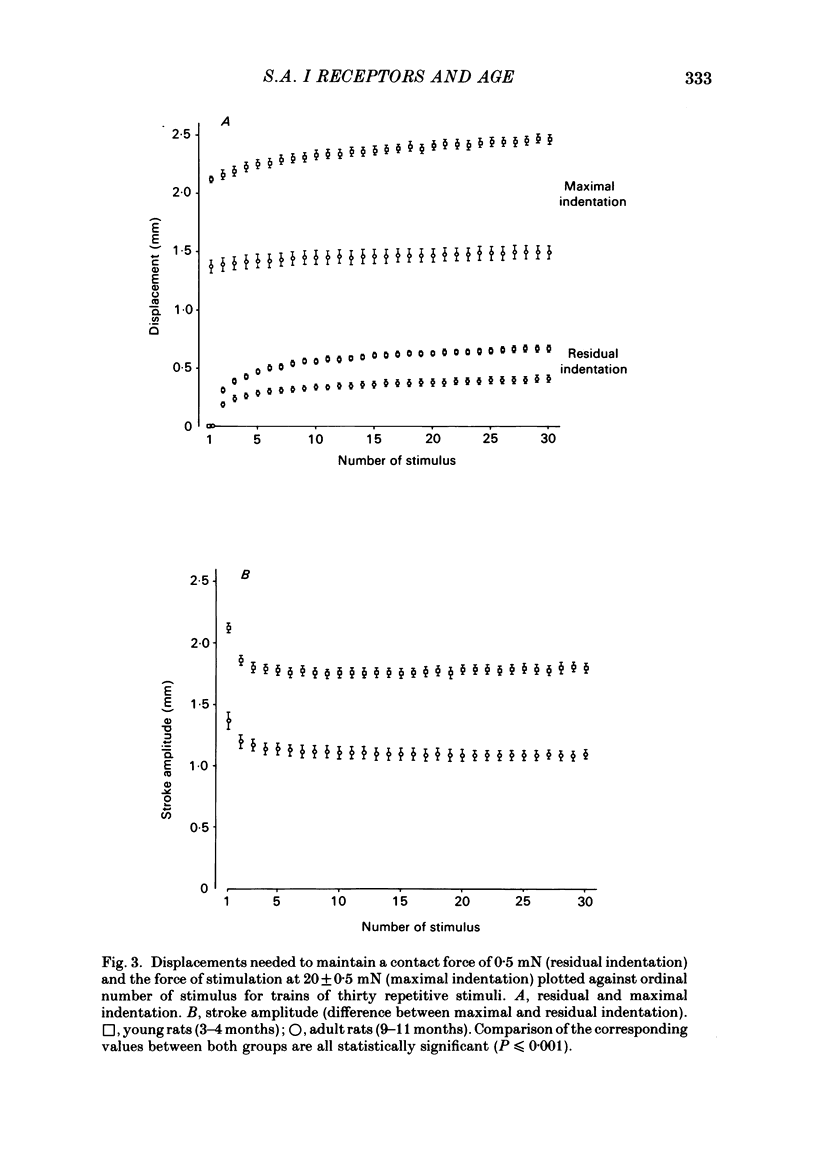
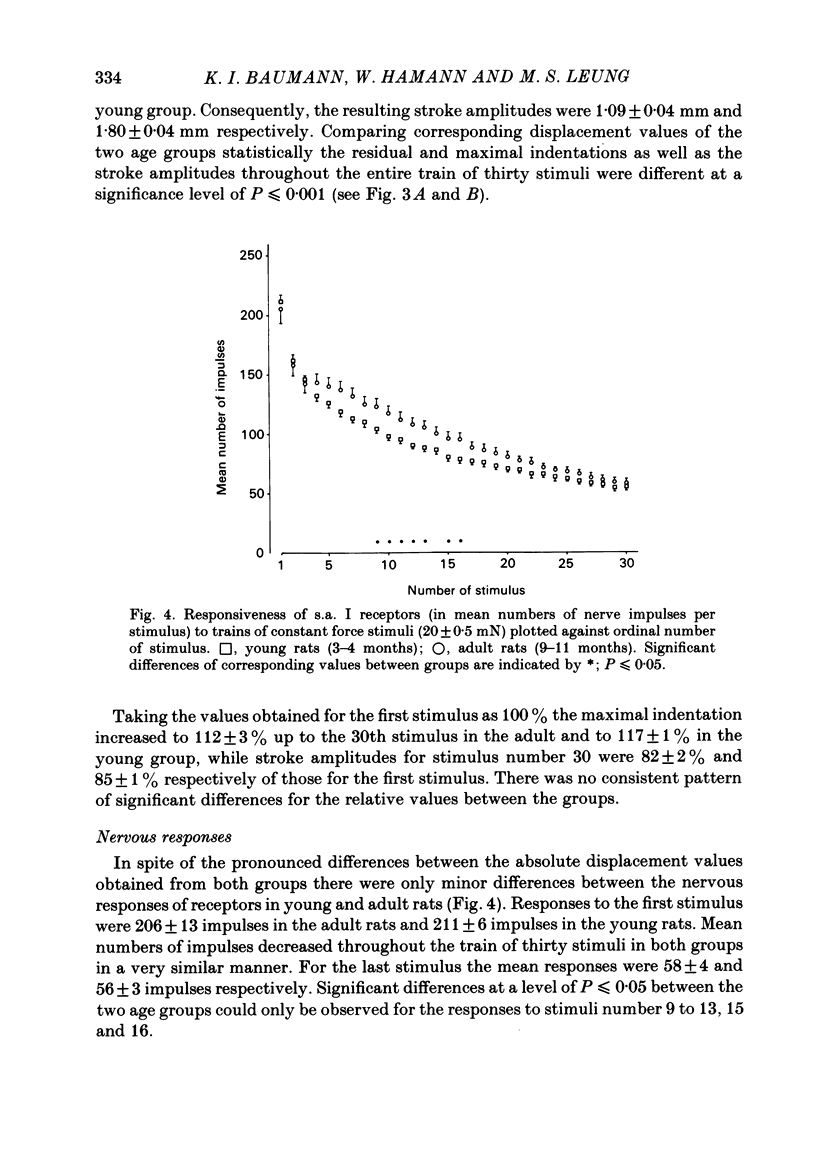
Slowly adapting type I (s.a. I) cutaneous mechanoreceptors were studied in young (3-4 months old) and adult (9-11 months old) rats. Trains of thirty repetitive mechanical stimuli with 0.1 s rise time, 1.9 s plateau phase, and 0.7 s interstimulus interval were applied. A feed-back mechanism maintained the force of stimulation at 20 mN during the plateau phases of stimuli and the contact force between stimuli at 0.5 mN. During the first few stimuli in a train residual indentation at contact force increased rapidly. Maximal indentation required to maintain the force of stimulation of 20 mN increased as well but to a smaller extent. Thus, the stroke amplitudes of individual stimuli decreased with increasing stimulus number. All displacement values in the group of adult rats were consistently reduced to 62 +/- 3% of the respective values in the group of young rats, indicating a linear decrease in skin compliance in the force range of 0.5-20 mN. Nervous responses to individual stimuli decreased from about 200 impulses for stimulus number 1 to about 60 impulses for stimulus number 30. Significant differences in the number of impulses between young and adult rats were observed from stimulus number 9 to number 16 only. It is concluded that the design of the s.a. I receptor allows maintained high tactile sensitivity in response to force-related stimuli irrespective of age-induced changes in mechanical properties of the skin and underlying tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agache P. G., Monneur C., Leveque J. L., De Rigal J. Mechanical properties and Young's modulus of human skin in vivo. Arch Dermatol Res. 1980;269(3):221–232. doi: 10.1007/BF00406415. [DOI] [PubMed] [Google Scholar]
- Barker D. J., Shepard P. D., McDermott K. L. Fatigue in cat facial mechanoreceptors. Neurosci Lett. 1982 May 28;30(2):117–122. doi: 10.1016/0304-3940(82)90282-8. [DOI] [PubMed] [Google Scholar]
- Baumann K. I., Cheng-Chew S. B., Hamann W., Leung M. S. Responsiveness and ultrastructure of slowly adapting type I cutaneous mechanoreceptors in vitamin A deficient rats. J Physiol. 1986 Feb;371:339–349. doi: 10.1113/jphysiol.1986.sp015979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle K. S., Montagna W. Aging model for unexposed human dermis. J Invest Dermatol. 1979 Jul;73(1):54–58. doi: 10.1111/1523-1747.ep12532762. [DOI] [PubMed] [Google Scholar]
- Catton W. T. Mechanoreceptor function. Physiol Rev. 1970 Jul;50(3):297–318. doi: 10.1152/physrev.1970.50.3.297. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., McNelly N. A., Hinds J. W. Variation in longevity of rats: evidence for a systematic increase in lifespan over time. Exp Aging Res. 1984 Autumn;10(3):137–140. doi: 10.1080/03610738408258556. [DOI] [PubMed] [Google Scholar]
- Daly C. H., Odland G. F. Age-related changes in the mechanical properties of human skin. J Invest Dermatol. 1979 Jul;73(1):84–87. doi: 10.1111/1523-1747.ep12532770. [DOI] [PubMed] [Google Scholar]
- Hamann W. C., Lee N. B. Sensitivity of touch corpuscles in vitamin A-deficient rats. Q J Exp Physiol. 1982 Apr;67(2):281–286. doi: 10.1113/expphysiol.1982.sp002636. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger L., Kenton B. Quantitative neural and psychophysical data for cutaneous mechanoreceptor function. Brain Res. 1973 Jan 15;49(1):1–24. doi: 10.1016/0006-8993(73)90398-3. [DOI] [PubMed] [Google Scholar]
- Lavker R. M. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979 Jul;73(1):59–66. doi: 10.1111/1523-1747.ep12532763. [DOI] [PubMed] [Google Scholar]
- Lindblom U. Properties of touch receptors in distal glabrous skin of the monkey. J Neurophysiol. 1965 Sep;28(5):966–985. doi: 10.1152/jn.1965.28.5.966. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Onodera K. Adaptation of the generator potential in the crayfish stretch receptors under constant length and constant tension. J Physiol. 1969 Jan;200(1):187–204. doi: 10.1113/jphysiol.1969.sp008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse C. A., Diamond J. A fluorescent microscopic study of the development of rat touch domes and their Merkel cells. Neuroscience. 1984 Feb;11(2):509–520. doi: 10.1016/0306-4522(84)90041-1. [DOI] [PubMed] [Google Scholar]
- Pubols B. H., Jr Factors affecting cutaneous mechanoreceptor response. I. Constant-force versus constant-displacement stimulation. J Neurophysiol. 1982 Mar;47(3):515–529. doi: 10.1152/jn.1982.47.3.515. [DOI] [PubMed] [Google Scholar]
- Ridge M. D., Wright V. Mechanical properties of skin: a bioengineering study of skin structure. J Appl Physiol. 1966 Sep;21(5):1602–1606. doi: 10.1152/jappl.1966.21.5.1602. [DOI] [PubMed] [Google Scholar]
- Verrillo R. T. Change in vibrotactile thresholds as a function of age. Sens Processes. 1979 Mar;3(1):49–59. [PubMed] [Google Scholar]


