Abstract
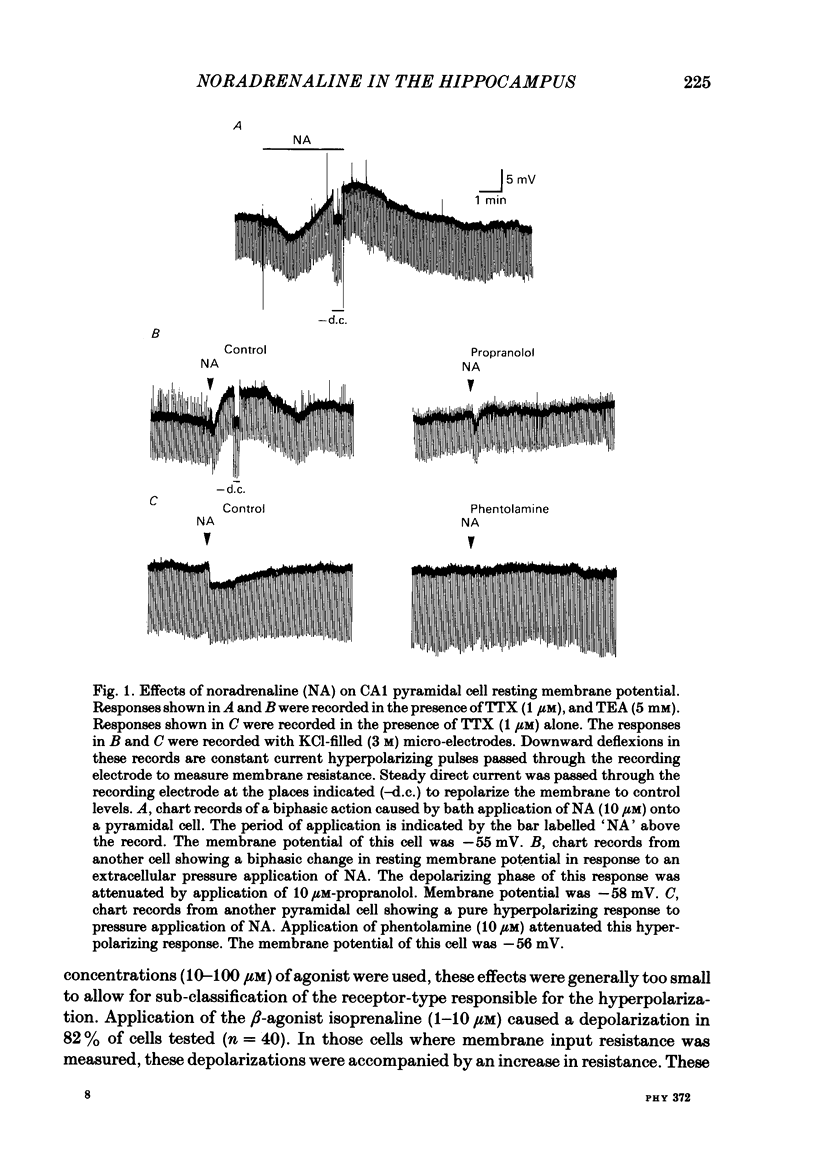
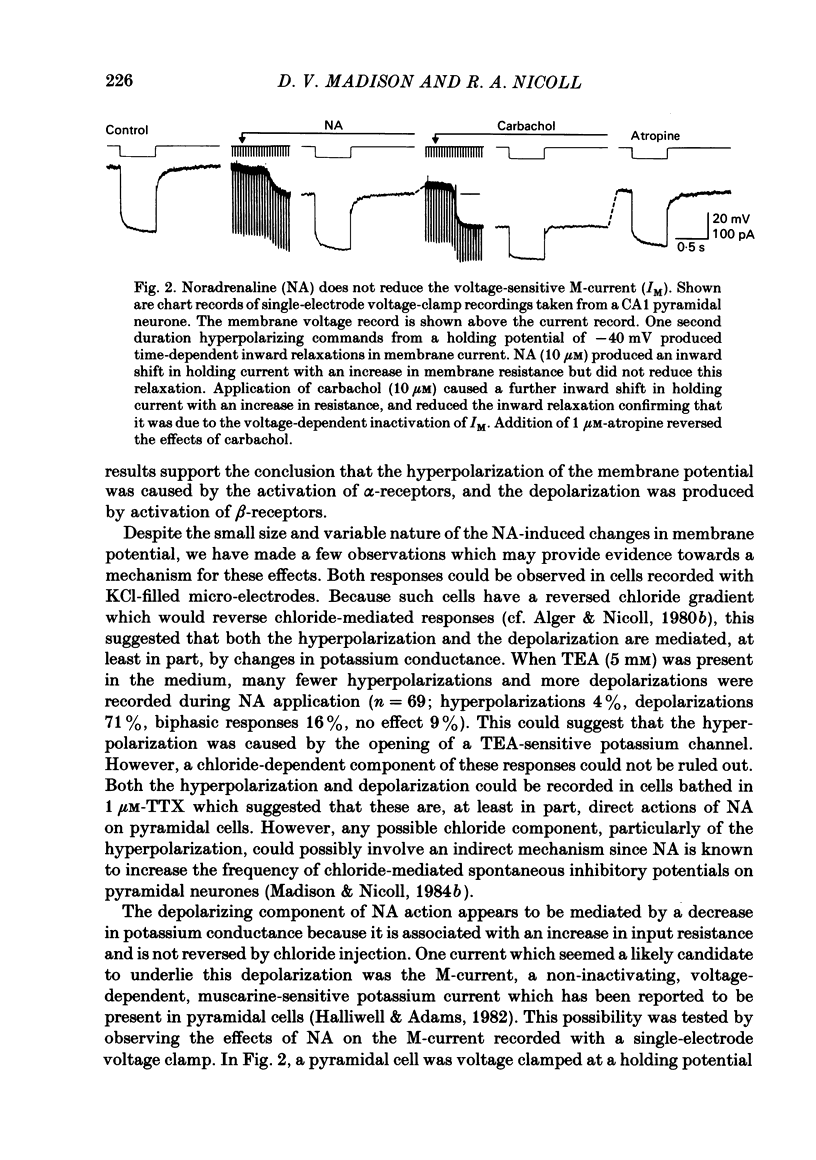
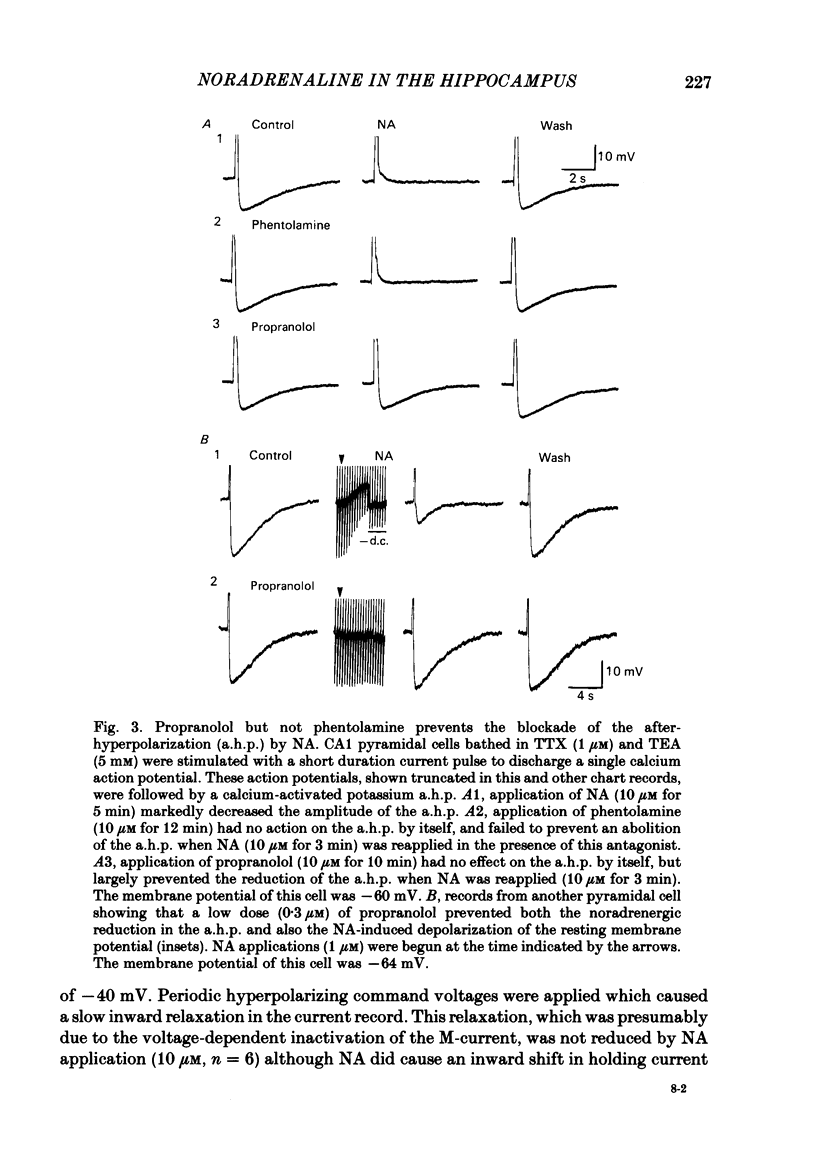
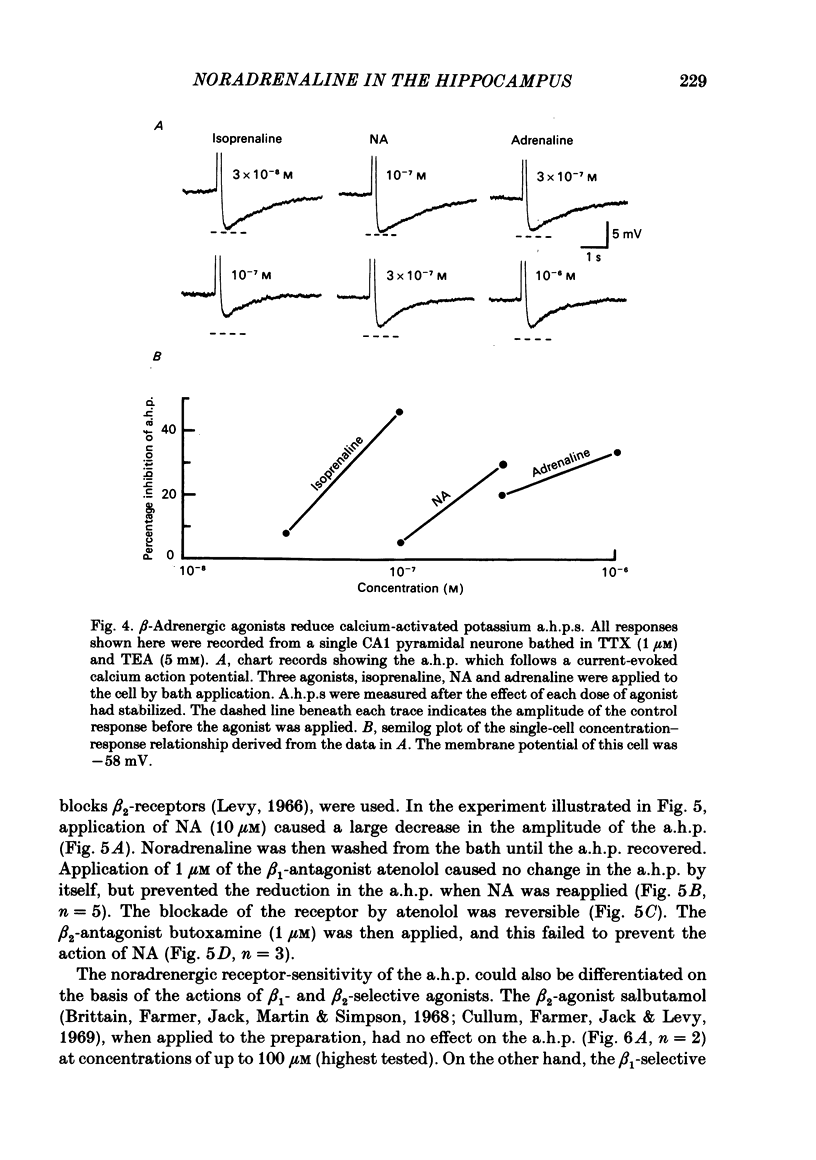
CA1 pyramidal neurones were studied in rat in vitro hippocampal slices using standard intracellular and single-electrode voltage-clamp recording techniques to examine the actions of noradrenaline (NA). NA had two different effects on the resting membrane potential of pyramidal neurones; either a hyperpolarization accompanied by a decrease in membrane input resistance, or less commonly, a depolarization accompanied by an increase in input resistance. In many cells, both effects, a hyperpolarization followed by a depolarization were observed. The depolarization was mediated by a noradrenergic beta-receptor. The hyperpolarization was more difficult to characterize, but may result from alpha-receptor activation. NA reduced the amplitude and duration of the slow calcium-activated potassium after-hyperpolarization (a.h.p.) that follows depolarization-induced action potentials. This action of NA was mediated by beta 1-noradrenergic receptors. NA, in the presence of tetrodotoxin and tetraethylammonium, reduced the a.h.p. without reducing the size of the calcium action potential which preceded it. This was unlike the action of the calcium channel blocker, cadmium, which reduced the calcium action potential and the a.h.p. in parallel. Furthermore, NA did not reduce the amplitude of calcium or barium currents recorded under voltage clamp after blockade of potassium currents. A functional consequence of this blockade of the calcium-activated a.h.p. was a reduction of the accommodation of action potential discharge such that the excitatory responses of the neurone to depolarizing stimuli, such as glutamate application or current passed through the recording electrode, were enhanced. We conclude that the effects of NA on calcium-activated potassium conductance and on resting membrane potential can interact to increase the signal-to-noise ratio of hippocampal pyramidal neurone responsiveness.
Full text
PDF




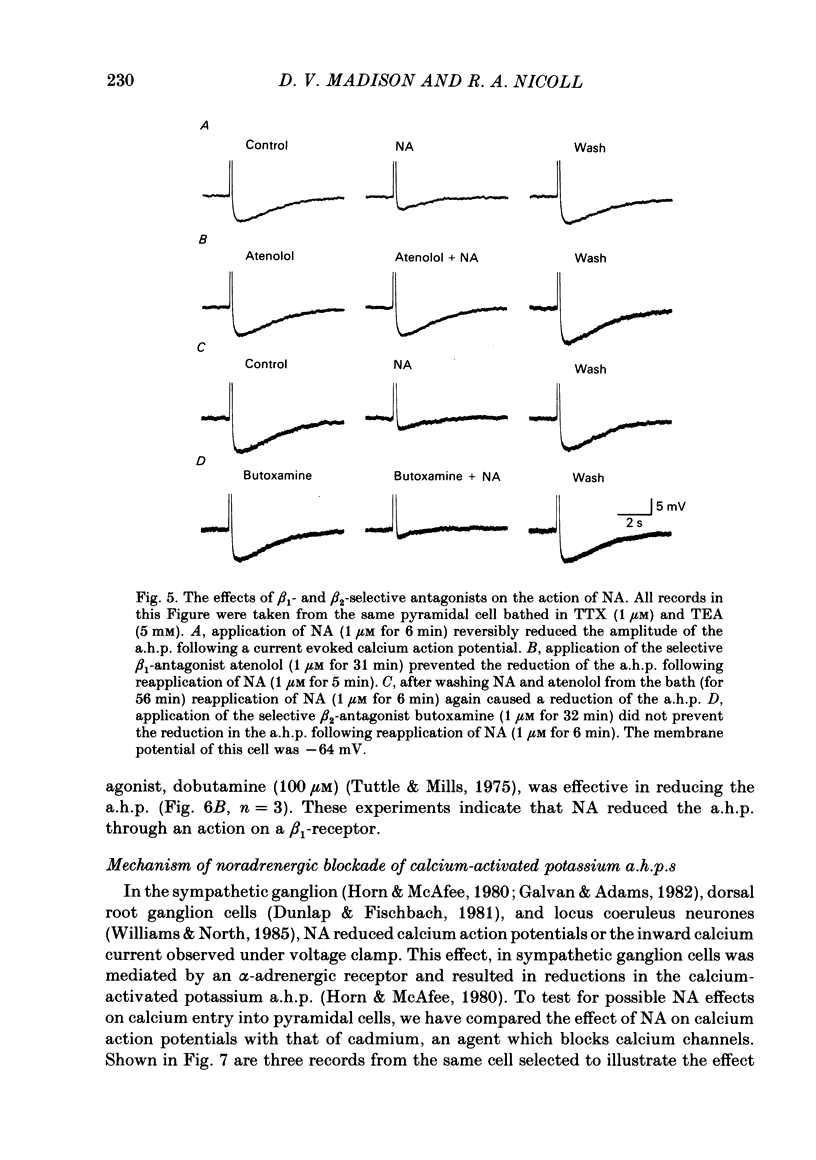
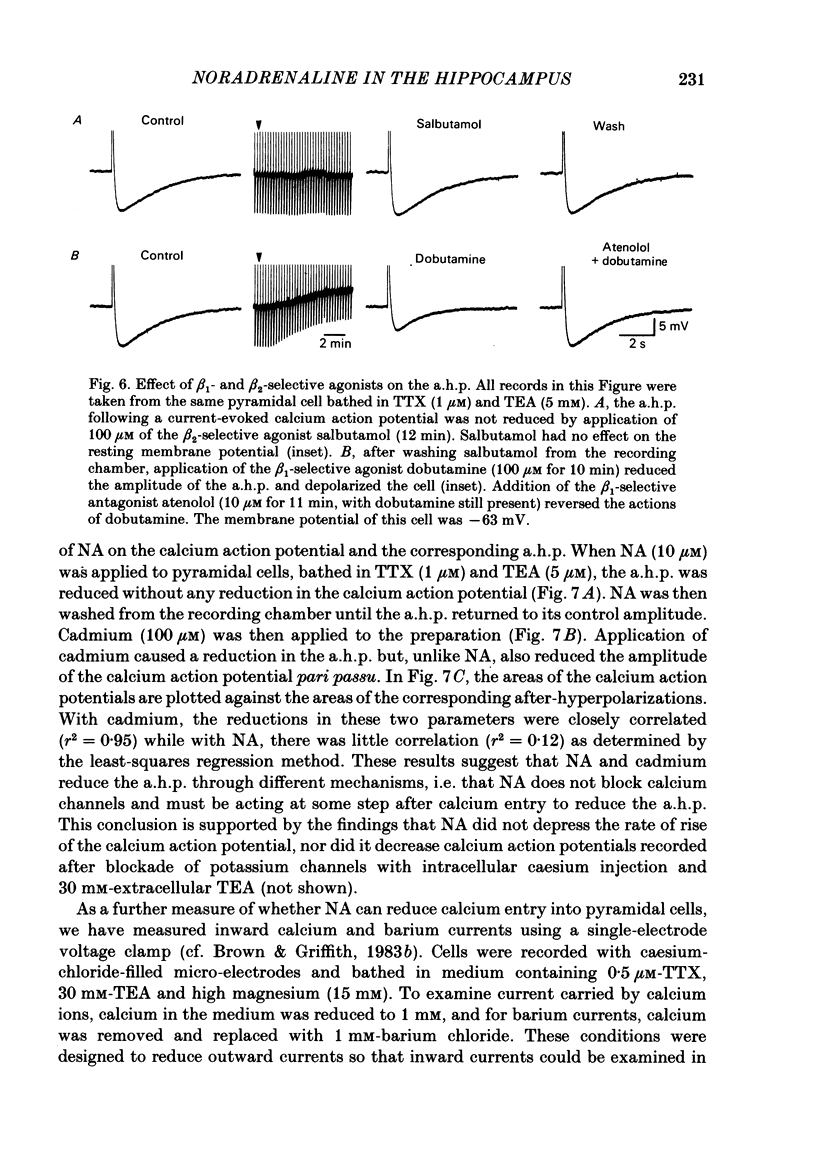
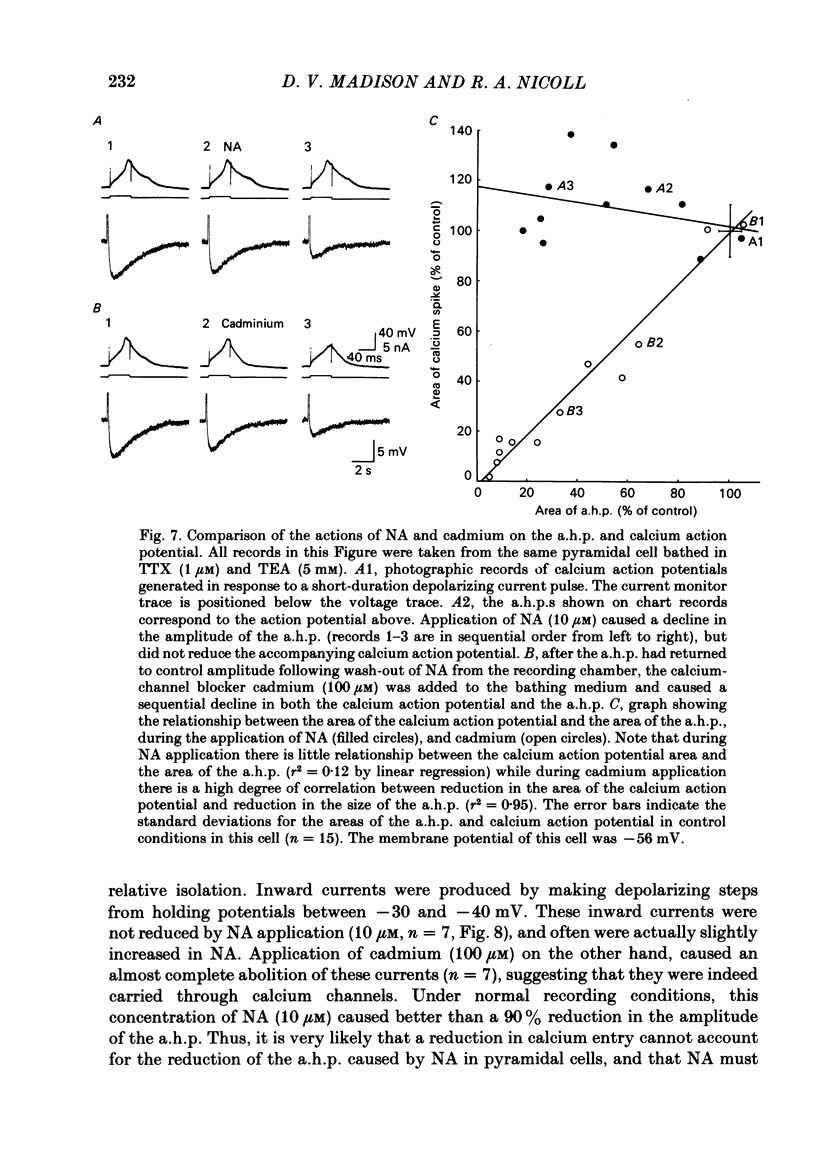
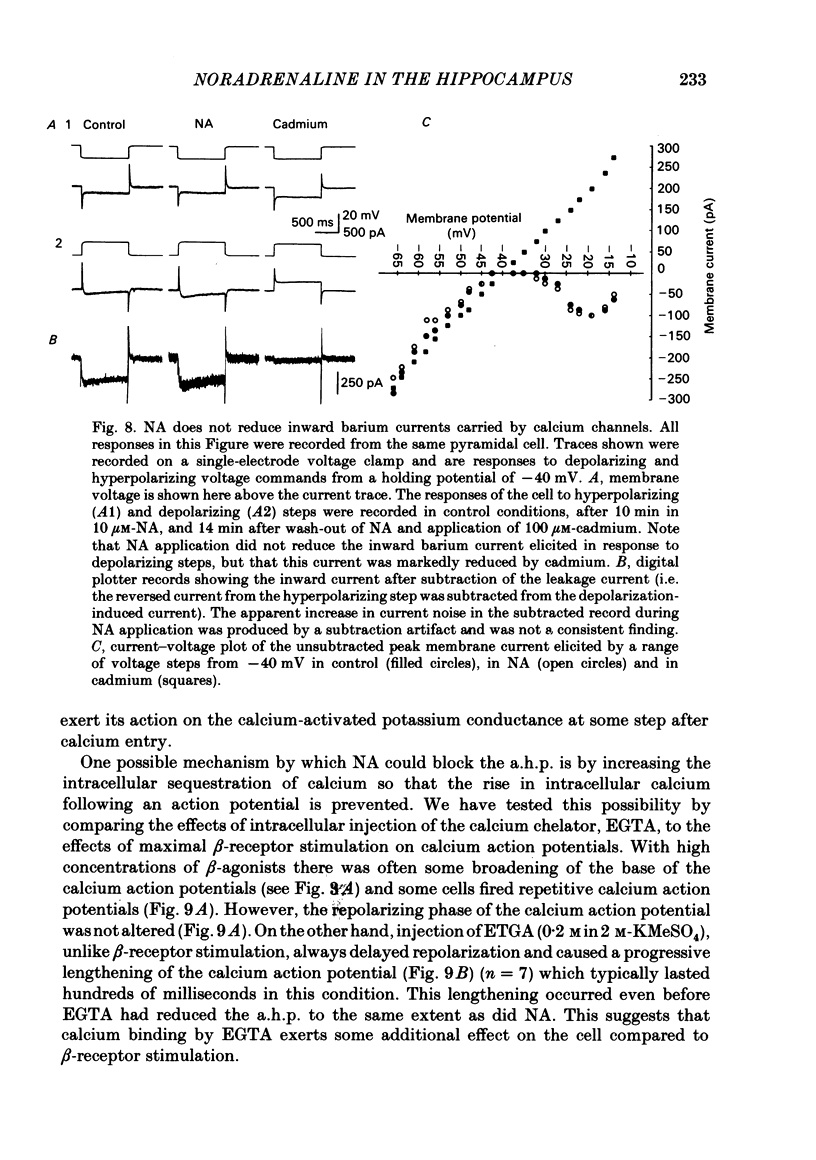








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenhoff J. B., Gruol D. L., Rivier J., Vale W., Siggins G. R. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983 Aug 26;221(4613):875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980 Oct 27;200(1):195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F. E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981 Aug;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F. E. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981 Aug;1(8):887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. M. The pharmacology of atenolol. Postgrad Med J. 1977;53 (Suppl 3):58–64. [PubMed] [Google Scholar]
- Basile A. S., Dunwiddie T. V. Norepinephrine elicits both excitatory and inhibitory responses from Purkinje cells in the in vitro rat cerebellar slice. Brain Res. 1984 Mar 26;296(1):15–25. doi: 10.1016/0006-8993(84)90507-9. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Straughan D. W. Micro-electrophoretic studies of neurones in the cat hippocampus. J Physiol. 1966 Mar;183(2):341–359. doi: 10.1113/jphysiol.1966.sp007869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F. E. Dynamic synaptic communication: finding the vocabulary. Brain Res. 1973 Nov 23;62(2):299–305. doi: 10.1016/0006-8993(73)90690-2. [DOI] [PubMed] [Google Scholar]
- Brittain R. T., Farmer J. B., Jack D., Martin L. E., Simpson W. T. Alpha-[(t-Butylamino)methyl]-4-hydroxy-m-xylene-alpha 1,alpha 3-diol (AH.3365): a selective beta-adrenergic stimulant. Nature. 1968 Aug 24;219(5156):862–863. doi: 10.1038/219862a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Depolarization of rat isolated superior cervical ganglia mediated by beta 2-adrenoceptors. Br J Pharmacol. 1983 Jun;79(2):429–439. doi: 10.1111/j.1476-5381.1983.tb11016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Persistent slow inward calcium current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:303–320. doi: 10.1113/jphysiol.1983.sp014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. A. Physiological role of a slow, voltage-sensitive, synaptic response mediated by an identified serotonin-containing neurone. Q J Exp Physiol. 1982 Jan;67(1):179–183. doi: 10.1113/expphysiol.1982.sp002612. [DOI] [PubMed] [Google Scholar]
- Cullum V. A., Farmer J. B., Jack D., Levy G. P. Salbutamol: a new, selective beta-adrenoceptive receptor stimulant. Br J Pharmacol. 1969 Jan;35(1):141–151. doi: 10.1111/j.1476-5381.1969.tb07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Henderson G., North R. A., Williams J. T. Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol. 1983 Dec;345:477–488. doi: 10.1113/jphysiol.1983.sp014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R., Hoffer B. J., Woodward D. J., Puro D. Interaction of norepinephrine with cerebellar activity evoked by mossy and climbing fibers. Exp Neurol. 1977 Apr;55(1):269–288. doi: 10.1016/0014-4886(77)90175-3. [DOI] [PubMed] [Google Scholar]
- Galvan M., Adams P. R. Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res. 1982 Jul 22;244(1):135–144. doi: 10.1016/0006-8993(82)90911-8. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Evidence for two types of afterhyperpolarization in CA1 pyramidal cells in the hippocampus. Brain Res. 1981 Feb 16;206(2):462–468. doi: 10.1016/0006-8993(81)90548-5. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. 1983 Mar 31-Apr 6Nature. 302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Haefely W. E. Effects of catecholamines in the cat superior cervical ganglion and their postulated rôle as physiological modulators of ganglionic transmission. Prog Brain Res. 1969;31:61–72. doi: 10.1016/S0079-6123(08)63228-8. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Herrling P. L. The membrane potential of cat hippocampal neurons recorded in vivo displays four different reaction-mechanisms to iontophoretically applied transmitter agonists. Brain Res. 1981 May 18;212(2):331–343. doi: 10.1016/0006-8993(81)90466-2. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Bloom F. E. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res. 1971 Feb 5;25(3):523–534. doi: 10.1016/0006-8993(71)90458-6. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Oliver A. P., Bloom F. E. Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther. 1973 Mar;184(3):553–569. [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Hughes I. E., Smith J. A. The stability of noradrenaline in physiological saline solutions. J Pharm Pharmacol. 1978 Feb;30(2):124–126. doi: 10.1111/j.2042-7158.1978.tb13179.x. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Nicoll R. A. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature. 1982 May 20;297(5863):227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T., Heggelund P. Single cell responses in cat visual cortex to visual stimulation during iontophoresis of noradrenaline. Exp Brain Res. 1982;45(3):317–327. doi: 10.1007/BF01208591. [DOI] [PubMed] [Google Scholar]
- Kayama Y., Negi T., Sugitani M., Iwama K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience. 1982 Mar;7(3):655–666. doi: 10.1016/0306-4522(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Langmoen I. A., Segal M., Andersen P. Mechanisms of norepinephrine actions on hippocampal pyramidal cells in vitro. Brain Res. 1981 Mar 16;208(2):349–362. doi: 10.1016/0006-8993(81)90563-1. [DOI] [PubMed] [Google Scholar]
- Lanthorn T., Storm J., Andersen P. Current-to-frequency transduction in CA1 hippocampal pyramidal cells: slow prepotentials dominate the primary range firing. Exp Brain Res. 1984;53(2):431–443. doi: 10.1007/BF00238173. [DOI] [PubMed] [Google Scholar]
- Levy B. The adrenergic blocking activity of N-tert.-butylmethoxamine (butoxamine). J Pharmacol Exp Ther. 1966 Mar;151(3):413–422. [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981 Jun 18;291(5816):554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Andersen R. A., Motter B. C. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci. 1981 Nov;1(11):1218–1225. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A. L., Hoffer B. J., Dunwiddie T. V. Noradrenergic responses in rat hippocampus: evidence for medication by alpha and beta receptors in the in vitro slice. Brain Res. 1981 Jun 9;214(1):113–126. doi: 10.1016/0006-8993(81)90442-x. [DOI] [PubMed] [Google Scholar]
- Mueller A. L., Palmer M. R., Hoffer B. J., Dunwiddie T. V. Hippocampal noradrenergic responses in vivo and in vitro. Characterization of alpha and beta components. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):259–266. doi: 10.1007/BF00501163. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Takaori S. Influence of norepinephrine-containing neurons derived from the locus coeruleus on lateral geniculate neuronal activities of cats. Brain Res. 1974 May 10;71(1):47–60. doi: 10.1016/0006-8993(74)90190-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yoshimura M., Shinnick-Gallagher P., Gallagher J. P., Akasu T. alpha 2 and alpha 1-Adrenoceptors mediate opposing actions on parasympathetic neurons. Brain Res. 1984 Dec 10;323(2):349–353. doi: 10.1016/0006-8993(84)90312-3. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Depression of calcium-dependent potassium conductance of guinea-pig myenteric neurones by muscarinic agonists. J Physiol. 1983 Sep;342:253–266. doi: 10.1113/jphysiol.1983.sp014849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhov N. A., Bragin A. G. Effects of norepinephrine and serotonin upon spontaneous activity and responses to mossy fiber stimulation of CA3 neurons in hippocampal slices. Brain Res. 1982 Dec 16;253(1-2):173–183. doi: 10.1016/0006-8993(82)90684-9. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A., Aghajanian G. K. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature. 1980 Oct 23;287(5784):731–734. doi: 10.1038/287731a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. I. Iontophoretic studies. Brain Res. 1974 May 31;72(1):79–97. doi: 10.1016/0006-8993(74)90652-0. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. II. Activation of the input pathway. Brain Res. 1974 May 31;72(1):99–114. doi: 10.1016/0006-8993(74)90653-2. [DOI] [PubMed] [Google Scholar]
- Segal M. Norepinephrine modulates reactivity of hippocampal cells to chemical stimulation in vitro. Exp Neurol. 1982 Jul;77(1):86–93. doi: 10.1016/0014-4886(82)90145-5. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of norepinephrine in the rat hippocampus: intracellular studies in the slice preparation. Brain Res. 1981 Feb 9;206(1):107–128. doi: 10.1016/0006-8993(81)90104-9. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Oliver A. P., Hoffer B. J., Bloom F. E. Cyclic adenosine monophosphate and norepinephrine: effects on transmembrane properties of cerebellar Purkinje cells. Science. 1971 Jan 15;171(3967):192–194. doi: 10.1126/science.171.3967.192. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Tuttle R. R., Mills J. Dobutamine: development of a new catecholamine to selectively increase cardiac contractility. Circ Res. 1975 Jan;36(1):185–196. doi: 10.1161/01.res.36.1.185. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A. Catecholamine inhibition of calcium action potentials in rat locus coeruleus neurones. Neuroscience. 1985 Jan;14(1):103–109. doi: 10.1016/0306-4522(85)90167-8. [DOI] [PubMed] [Google Scholar]
- Woodward D. J., Moises H. C., Waterhouse B. D., Hoffer B. J., Freedman R. Modulatory actions of norepinephrine in the central nervous system. Fed Proc. 1979 Jun;38(7):2109–2116. [PubMed] [Google Scholar]


