Abstract
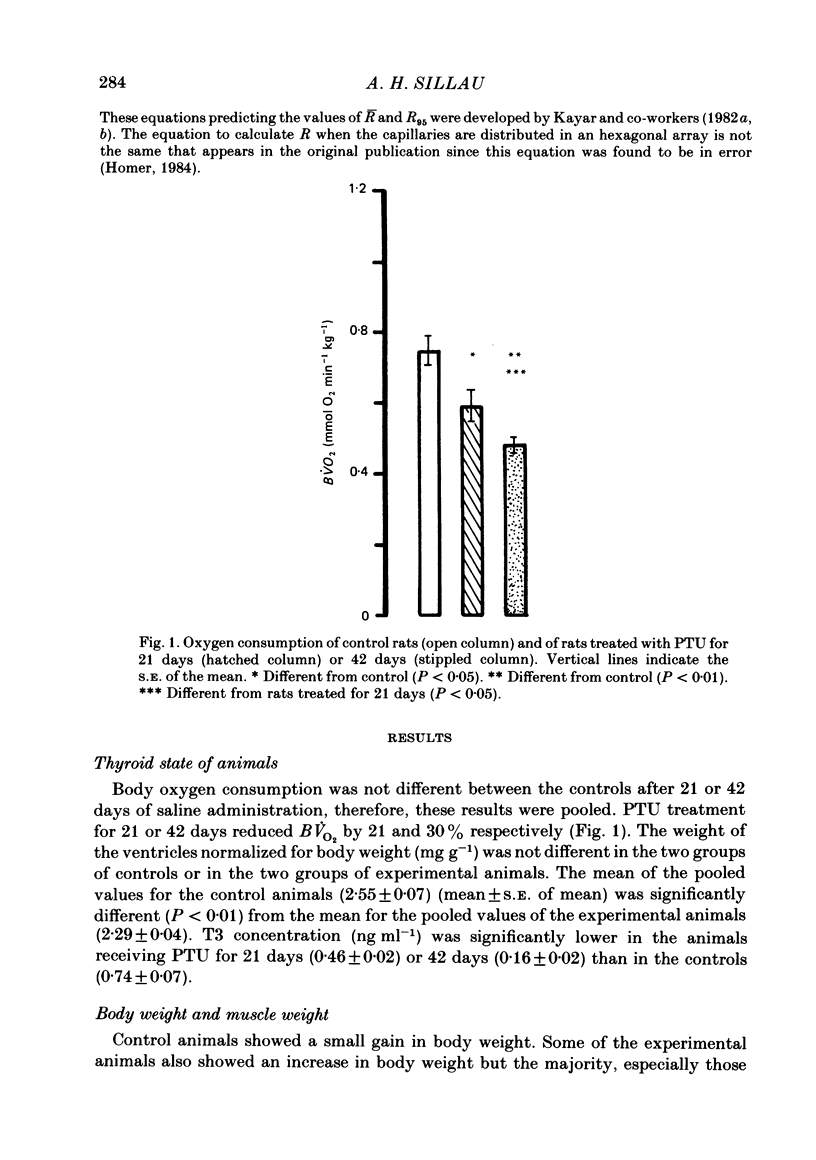
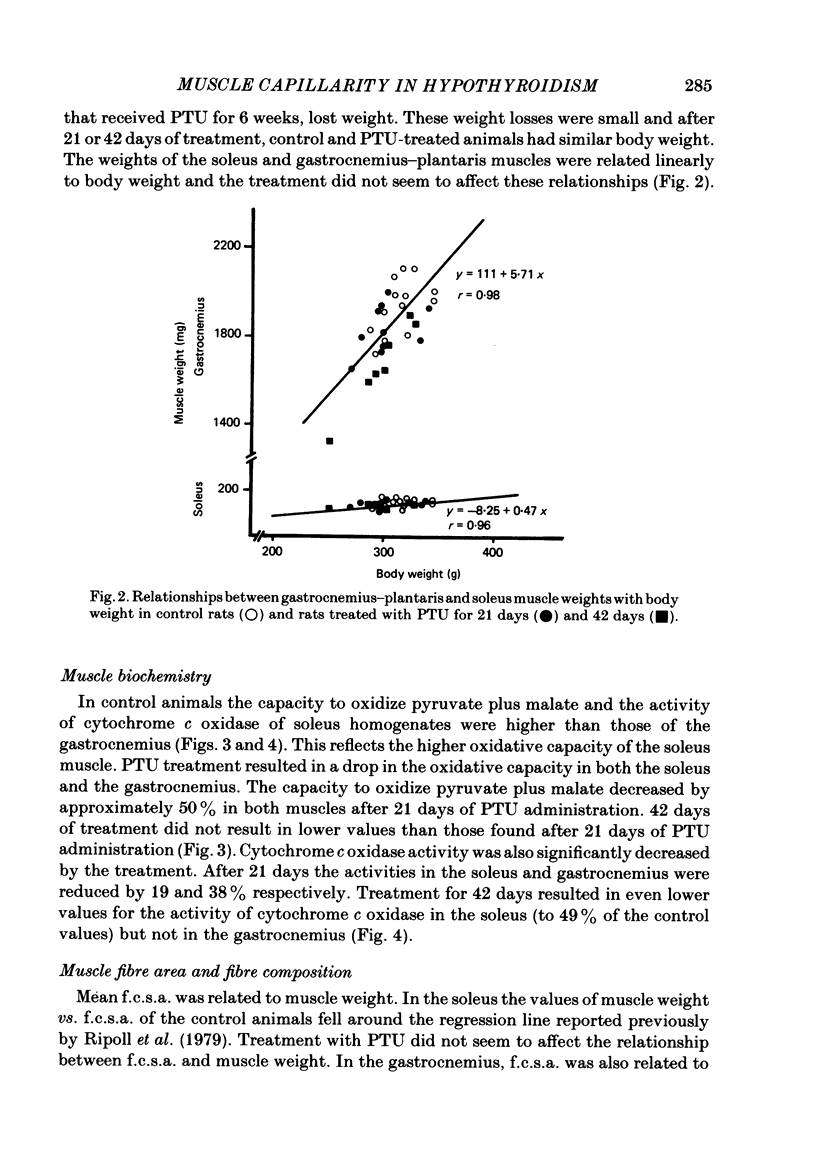
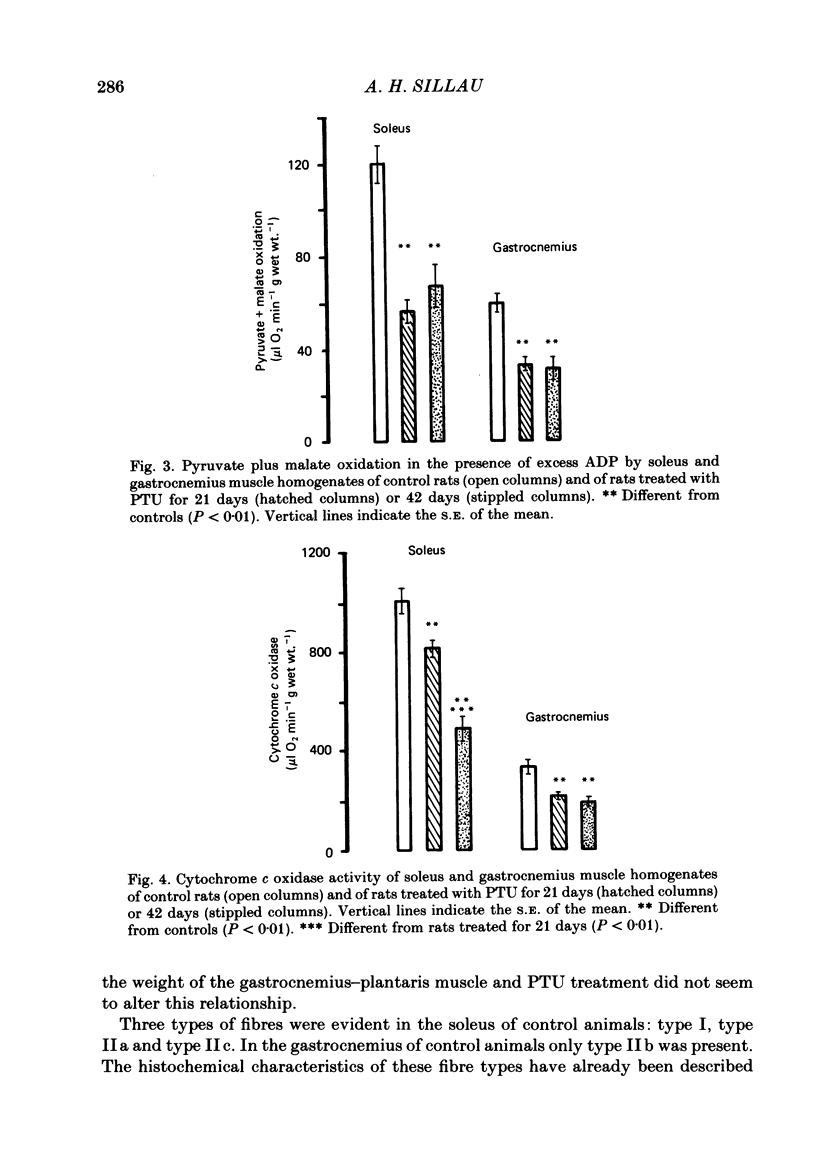
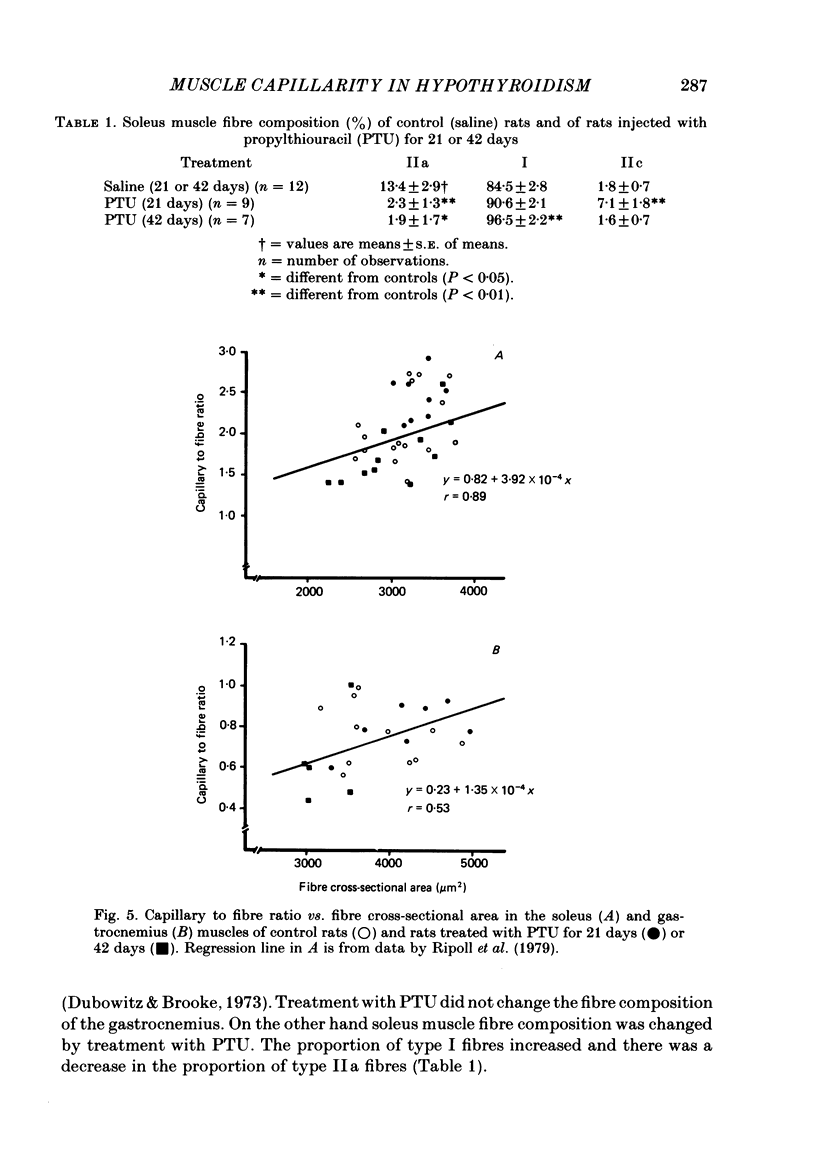
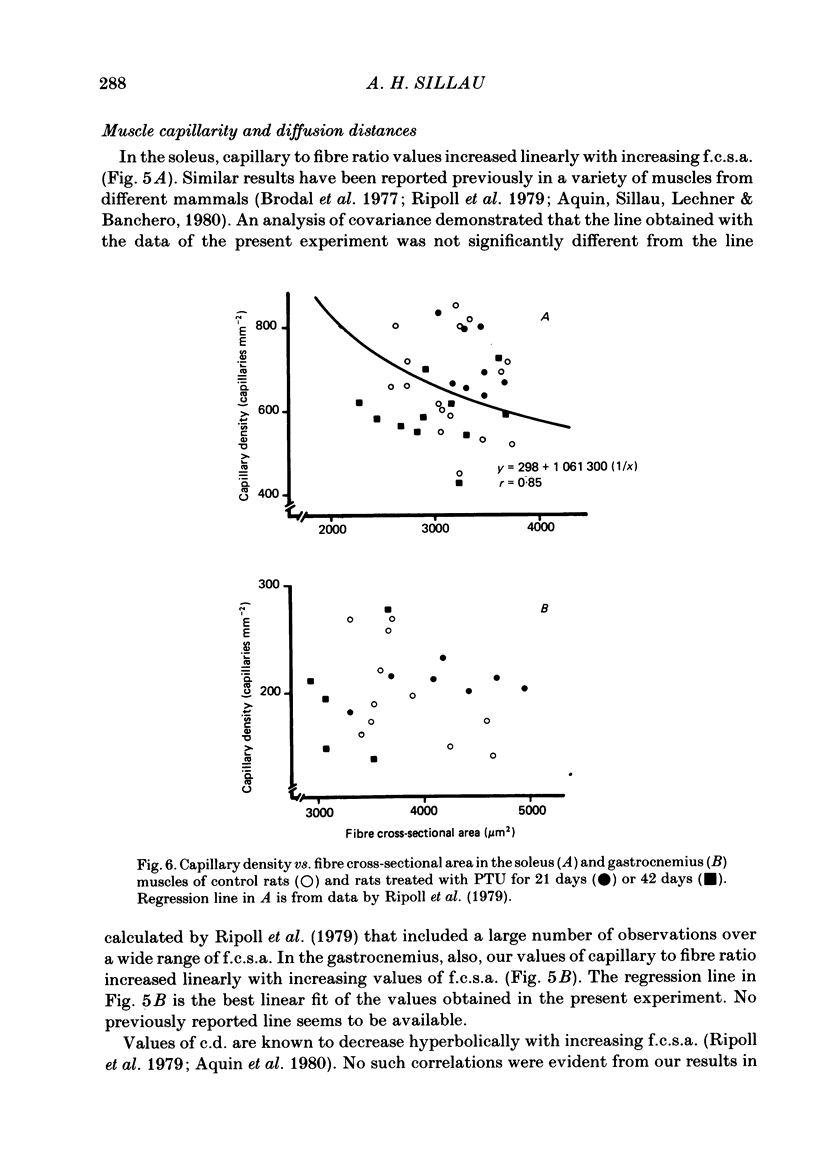
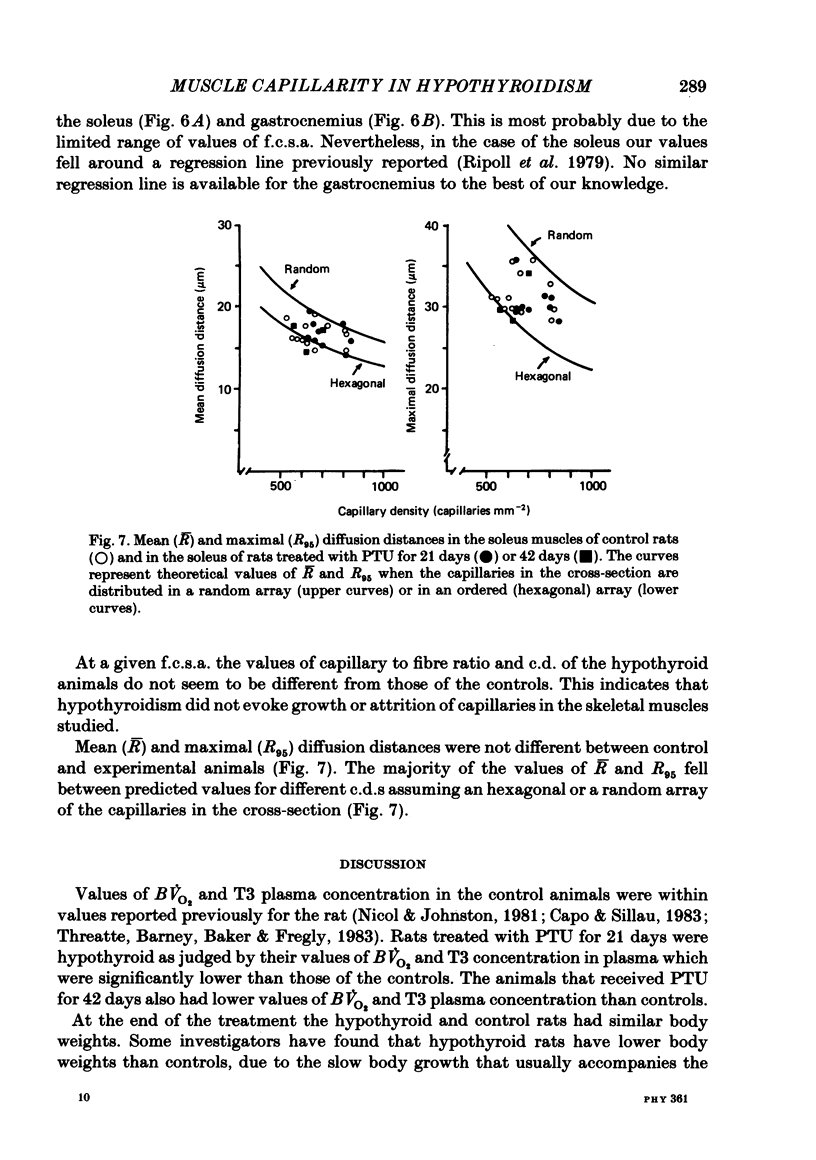
Muscle capillarity, mean and maximal diffusion distances and muscle fibre composition were evaluated in frozen sections stained for myosin ATPase of the soleus and the white area of the gastrocnemius medial head (gastrocnemius) of rats made hypothyroid by the injection of propylthiouracil (PTU) (50 mg kg-1) every day for 21 or 42 days. Oxygen consumption in the presence of excess ADP and Pi with pyruvate plus malate as substrates and the activity of cytochrome c oxidase were measured in muscle homogenates. Treatment with PTU decreased body oxygen consumption and the concentration of triiodothyronine in plasma. The capacity of the soleus and gastrocnemius muscles' homogenates to oxidize pyruvate plus malate and their cytochrome c oxidase activity were reduced after 21 or 42 days of treatment with PTU. Fibre composition in the soleus muscle was changed by treatment with PTU. There was a decrease in the proportion of type IIa or fast glycolytic oxidative fibres and an increase in type I or slow oxidative fibres. After 21 days of PTU administration there was also an increase in the proportion of fibres classified as IIc. The changes in fibre composition are believed to be the result of changes in the types of myosin synthesized by the fibres. Therefore, the fibres classified as IIc are, most probably, IIa fibres in the process of changing their myosin to that of the type I fibres. No changes in fibre composition were evident in the white area of the gastrocnemius medial head, an area made up of IIb or fast glycolytic fibres. The indices of capillarity: capillary density and capillary to fibre ratio, as well as mean and maximal diffusion distances from the capillaries, were not changed by the treatment with PTU in the muscles studied. The lack of changes in capillarity in spite of significant changes in oxidative capacity indicates that in skeletal muscle capillarity is not necessarily related to the oxidative capacity of the fibres.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquin L., Sillau A. H., Lechner A. J., Banchero N. Growth and skeletal muscle microvascularity in the guinea pig. Microvasc Res. 1980 Jul;20(1):41–50. doi: 10.1016/0026-2862(80)90018-7. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Hooker A. M., Campbell P. J., Lewis R. E. Enzyme changes in neonatal skeletal muscle: effect of thyroid deficiency. Am J Physiol. 1978 Sep;235(3):C97–102. doi: 10.1152/ajpcell.1978.235.3.C97. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Hooker A. M., Herrick R. E., Schrader L. F. Respiratory capacity and glycogen depletion in thyroid-deficient muscle. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jul;49(1):102–106. doi: 10.1152/jappl.1980.49.1.102. [DOI] [PubMed] [Google Scholar]
- Banchero N. Capillary density of skeletal muscle in dogs exposed to simulated altitude (38556). Proc Soc Exp Biol Med. 1975 Feb;148(2):435–439. doi: 10.3181/00379727-148-38556. [DOI] [PubMed] [Google Scholar]
- Brodal P., Ingjer F., Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 1977 Jun;232(6):H705–H712. doi: 10.1152/ajpheart.1977.232.6.H705. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Cotter M. A., Hudlická O., Vrbová G. The effects of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflugers Arch. 1976 Feb 24;361(3):241–250. doi: 10.1007/BF00587288. [DOI] [PubMed] [Google Scholar]
- Capó L. A., Sillau A. H. The effect of hyperthyroidism on capillarity and oxidative capacity in rat soleus and gastrocnemius muscles. J Physiol. 1983 Sep;342:1–14. doi: 10.1113/jphysiol.1983.sp014835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Frey H. M. Peripheral circulatory and metabolic consequences of thyrotoxicosis. I. Blood flow and oxygen consumption of resting and working skeletal muscle in experimental thyrotoxicosis in the dog. Scand J Clin Lab Invest. 1967;19(1):4–14. doi: 10.3109/00365516709093476. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Ianuzzo C. D. Hormonal deficiencies and the metabolic adaptations of rats to training. Am J Physiol. 1972 Aug;223(2):278–282. doi: 10.1152/ajplegacy.1972.223.2.278. [DOI] [PubMed] [Google Scholar]
- Gustafsson R., Tata J. R., Lindberg O., Ernster L. The relationship between the structure and activity of rat skeletal muscle mitochondria after thyroidectomy and thyroid hormone treatment. J Cell Biol. 1965 Aug;26(2):555–578. doi: 10.1083/jcb.26.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer L. D. Moments of distributions of distances to the nearest capillary in tissue. Microvasc Res. 1984 Jan;27(1):114–116. doi: 10.1016/0026-2862(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Hudlická O. Growth of capillaries in skeletal and cardiac muscle. Circ Res. 1982 Apr;50(4):451–461. doi: 10.1161/01.res.50.4.451. [DOI] [PubMed] [Google Scholar]
- IINO S., YAMADA T., GREER M. A. Effect of graded doses of propylthiouracil on biosynthesis of thyroid hormones. Endocrinology. 1961 Apr;68:582–588. doi: 10.1210/endo-68-4-582. [DOI] [PubMed] [Google Scholar]
- Ianuzzo C. D., Chen V., O'Brien P., Keens T. G. Effect of experimental dysthyroidism on the enzymatic character of the diaphragm. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):117–121. doi: 10.1152/jappl.1984.56.1.117. [DOI] [PubMed] [Google Scholar]
- Ianuzzo D., Patel P., Chen V., O'Brien P., Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature. 1977 Nov 3;270(5632):74–76. doi: 10.1038/270074a0. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Olmo J. L., Mastaglia F. L. Changes in histochemical profile of rat respiratory muscles in hypo- and hyperthyroidism. Q J Exp Physiol. 1983 Jan;68(1):1–13. doi: 10.1113/expphysiol.1983.sp002689. [DOI] [PubMed] [Google Scholar]
- KONTOS H. A., SHAPIRO W., MAUCK H. P., Jr, RICHARDSON D. W., PATTERSON J. L., Jr, SHARPE A. R., Jr MECHANISM OF CERTAIN ABNORMALITIES OF THE CIRCULATION TO THE LIMBS IN THYROTOXICOSIS. J Clin Invest. 1965 Jun;44:947–956. doi: 10.1172/JCI105212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayar S. R., Archer P. G., Lechner A. J., Banchero N. The closest-individual method in the analysis of the distribution of capillaries. Microvasc Res. 1982 Nov;24(3):326–341. doi: 10.1016/0026-2862(82)90020-6. [DOI] [PubMed] [Google Scholar]
- Kayar S. R., Lechner A. J., Banchero N. The distribution of diffusion distances in the gastrocnemius muscle of various mammals during maturation. Pflugers Arch. 1982 Aug;394(2):124–129. doi: 10.1007/BF00582913. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Maxwell L. C., White T. P., Faulkner J. A. Oxidative capacity, blood flow, and capillarity of skeletal muscles. J Appl Physiol Respir Environ Exerc Physiol. 1980 Oct;49(4):627–633. doi: 10.1152/jappl.1980.49.4.627. [DOI] [PubMed] [Google Scholar]
- ROMANUL F. C. CAPILLARY SUPPLY AND METABOLISM OF MUSCLE FIBERS. Arch Neurol. 1965 May;12:497–509. doi: 10.1001/archneur.1965.00460290053007. [DOI] [PubMed] [Google Scholar]
- Ripoll E., Sillau A. H., Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch. 1979 Jun 12;380(2):153–158. doi: 10.1007/BF00582151. [DOI] [PubMed] [Google Scholar]
- Sillau A. H., Aquin L., Bui M. V., Banchero N. Chronic hypoxia does not affect guinea pig skeletal muscle capillarity. Pflugers Arch. 1980 Jul;386(1):39–45. doi: 10.1007/BF00584185. [DOI] [PubMed] [Google Scholar]
- Sillau A. H., Aquin L., Lechner A. J., Bui M. V., Banchero N. Increased capillary supply in skeletal muscle of guinea pigs acclimated to cold. Respir Physiol. 1980 Dec;42(3):233–245. doi: 10.1016/0034-5687(80)90117-6. [DOI] [PubMed] [Google Scholar]
- Sillau A. H., Banchero N. Visualization of capillaries in skeletal muscle by the ATPase reaction. Pflugers Arch. 1977 Jul 19;369(3):269–271. doi: 10.1007/BF00582194. [DOI] [PubMed] [Google Scholar]
- Spaulding S. W., Noth R. H. Thyroid-catecholamine interactions. Med Clin North Am. 1975 Sep;59(5):1123–1131. doi: 10.1016/s0025-7125(16)31962-9. [DOI] [PubMed] [Google Scholar]
- Stoffer S. S., Jiang N. S., Gorman C. A., Pikler G. M. Plasma catecholamines in hypothyroidism and hyperthyroidism. J Clin Endocrinol Metab. 1973 Mar;36(3):587–589. doi: 10.1210/jcem-36-3-587. [DOI] [PubMed] [Google Scholar]
- Threatte R. M., Barney C. C., Baker S. P., Fregly M. J. Dependence of beta-adrenergic responsiveness on thyroid state of male rats. Clin Exp Pharmacol Physiol. 1983 Mar-Apr;10(2):101–114. doi: 10.1111/j.1440-1681.1983.tb00176.x. [DOI] [PubMed] [Google Scholar]
- VALDIVIA E. Total capillary bed in striated muscles of guinea pigs native to the Peruvian mountains. Am J Physiol. 1958 Sep;194(3):585–589. doi: 10.1152/ajplegacy.1958.194.3.585. [DOI] [PubMed] [Google Scholar]
- Wiles C. M., Young A., Jones D. A., Edwards R. H. Muscle relaxation rate, fibre-type composition and energy turnover in hyper- and hypo-thyroid patients. Clin Sci (Lond) 1979 Oct;57(4):375–384. doi: 10.1042/cs0570375. [DOI] [PubMed] [Google Scholar]
- Winder W. W., Baldwin K. M., Terjung R. L., Holloszy J. O. Effects of thyroid hormone administration on skeletal muscle mitochondria. Am J Physiol. 1975 May;228(5):1341–1345. doi: 10.1152/ajplegacy.1975.228.5.1341. [DOI] [PubMed] [Google Scholar]
- Winder W. W., Holloszy J. O. Response of mitochondria of different types of skeletal muscle to thyrotoxicosis. Am J Physiol. 1977 May;232(5):C180–C184. doi: 10.1152/ajpcell.1977.232.5.C180. [DOI] [PubMed] [Google Scholar]
- ZSOTER T., TOM H., CHAPPEL C. EFFECT OF THYROID HORMONES ON VASCULAR RESPONSE. J Lab Clin Med. 1964 Sep;64:433–441. [PubMed] [Google Scholar]


