Abstract
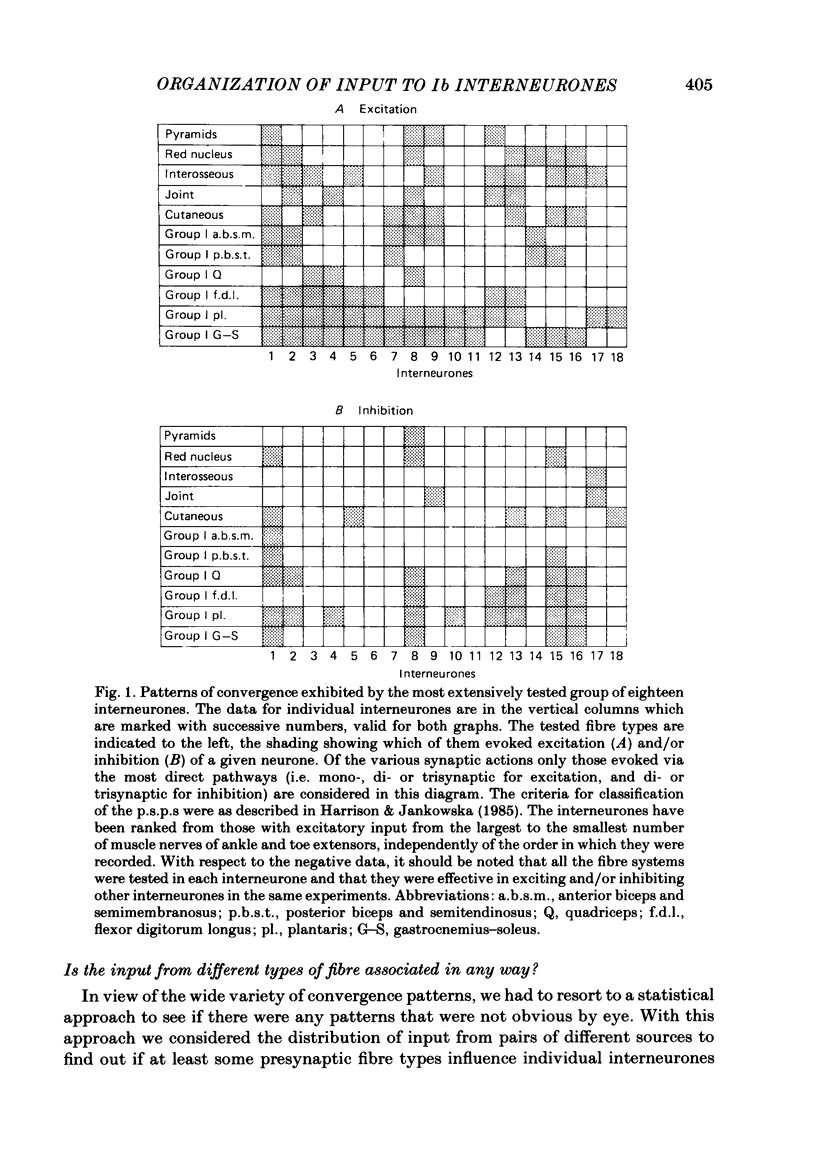
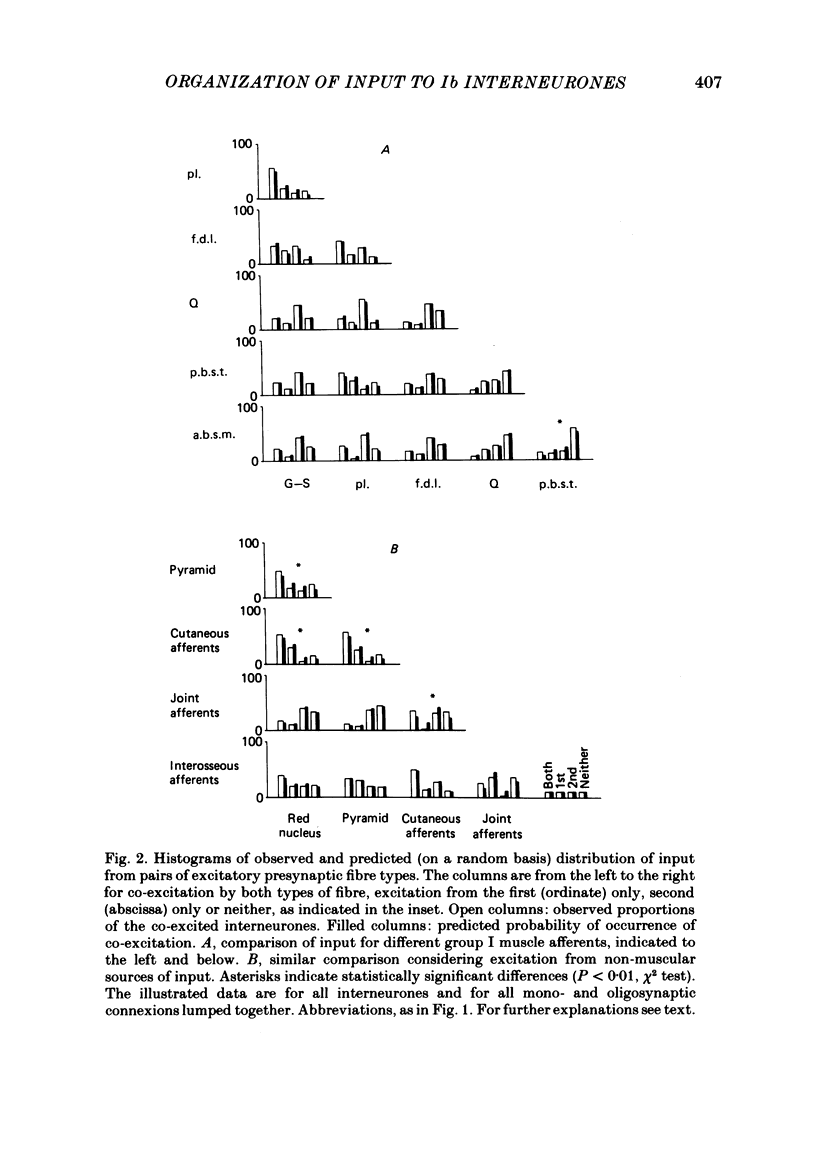
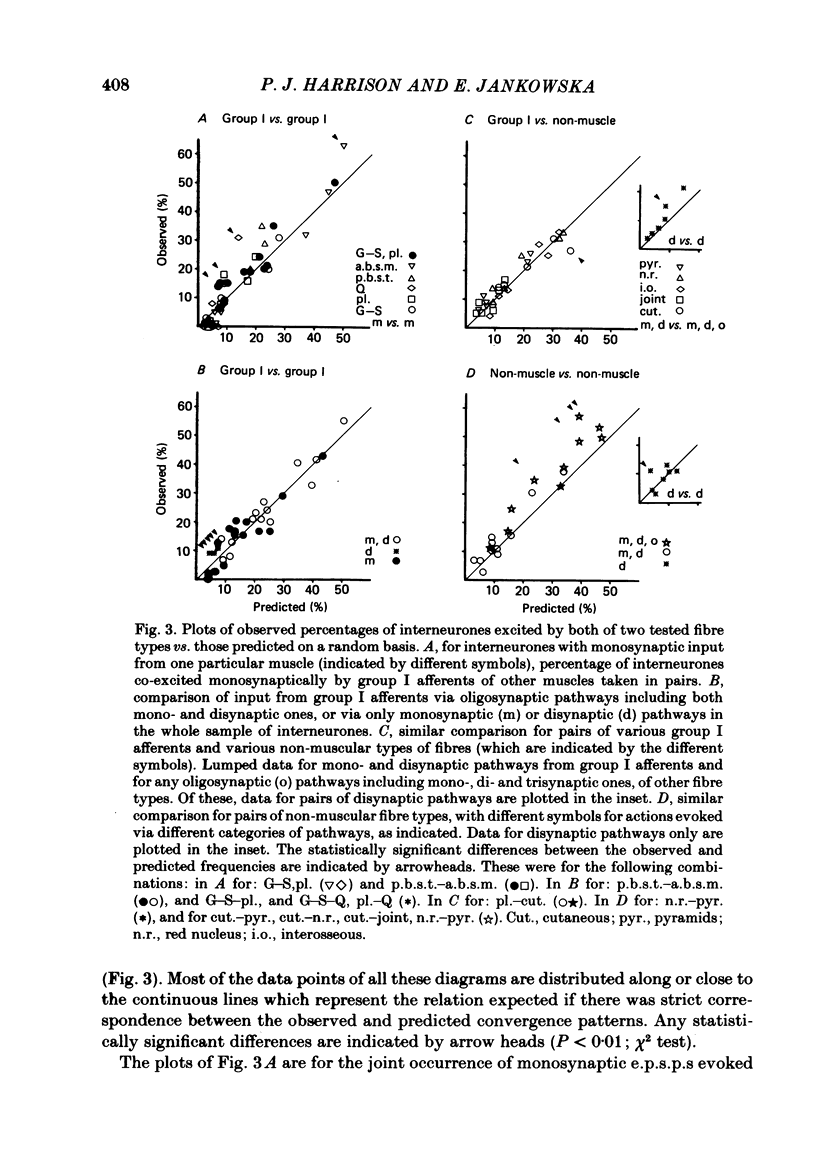
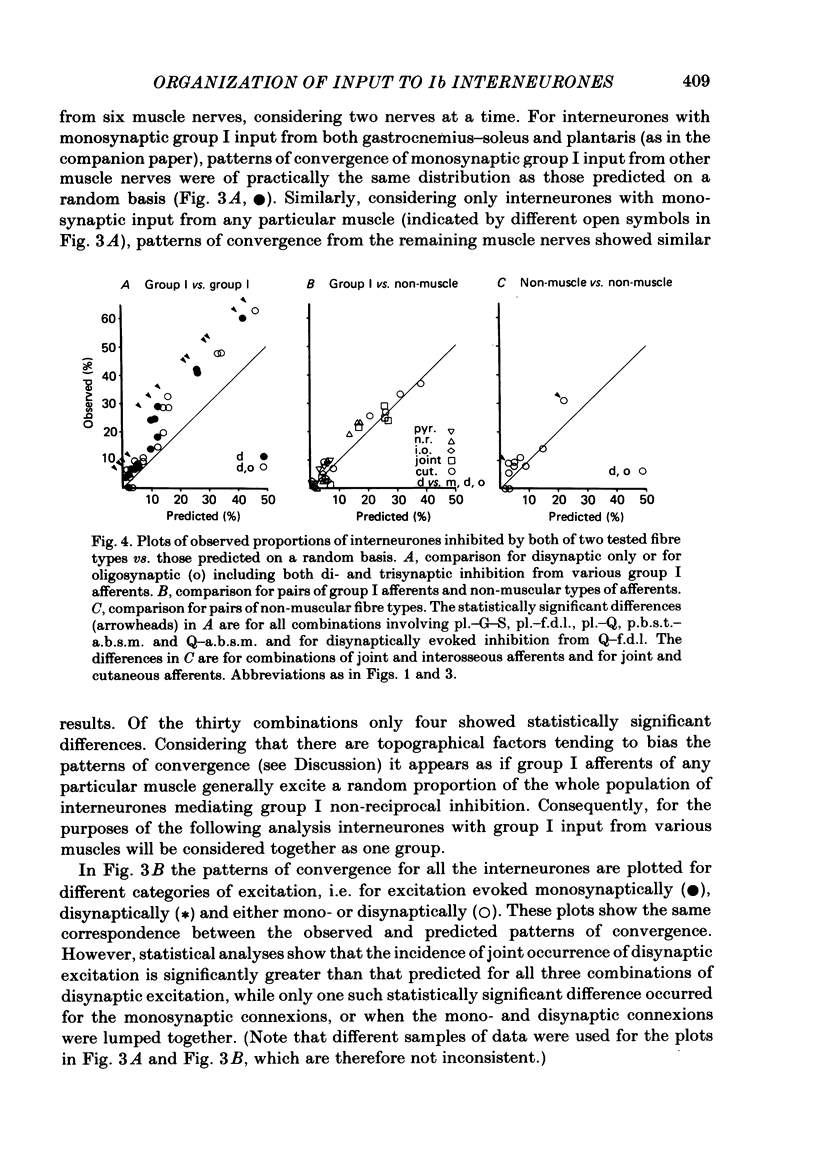
Patterns of convergence of different presynaptic fibre types onto interneurones mediating non-reciprocal inhibition of motoneurones have been studied in order to investigate to what extent the population of these interneurones is homogeneous or can be divided into subgroups on the basis of their input. In a sample of interneurones, all of which were interposed in pathways from the group I afferents of one group of muscles (triceps surae and plantaris), individual interneurones exhibited a wide variety of convergence patterns. Some interneurones were influenced by only a few types of afferent or descending fibre systems whereas others were influenced by many. Furthermore, various fibre systems excited and/or inhibited individual interneurones in different combinations. While there appeared to be too many patterns of convergence to allow any simple classification into a few distinct groups of interneurones, two possibilities were considered. One was that certain presynaptic fibre types influence individual interneurones in preferred combinations. The other was that they converge entirely at random. To investigate this, the frequencies of convergence of various pairs of fibre types were predicted assuming that each of them influences a proportion of the interneurones independently of other sources. Generally, there was close correspondence between such predicted and observed frequencies of occurrence of tested combinations of input. These findings are thus compatible with an organization whereby individual presynaptic fibres innervate a random sample of the population of interneurones. Deviations from the predicted incidence of convergence patterns were found primarily for synaptic actions mediated di- or oligosynaptically and are attributed to a consequence of convergence at the pre-interneuronal level. A particular consequence of such an organization is that interneurones in pathways of non-reciprocal inhibition are shared by afferents of different muscles in a continuum of combinations. The functional implications of this arrangement are discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brink E., Jankowska E., McCrea D. A., Skoog B. Inhibitory interactions between interneurones in reflex pathways from group Ia and group Ib afferents in the cat. J Physiol. 1983 Oct;343:361–373. doi: 10.1113/jphysiol.1983.sp014897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ib afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1979 Nov;296:215–226. doi: 10.1113/jphysiol.1979.sp013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska J., Jankowska E., Sybirska E. Common interneurones in reflex pathways from group 1a and 1b afferents of knee flexors and extensors in the cat. J Physiol. 1981 Jan;310:367–380. doi: 10.1113/jphysiol.1981.sp013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E., Johannisson T. Shared reflex pathways of group I afferents of different cat hind-limb muscles. J Physiol. 1983 May;338:113–128. doi: 10.1113/jphysiol.1983.sp014664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985 Apr;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. IV. Effects on interneurones. Exp Brain Res. 1972;15(1):54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol. 1983 Sep;342:145–159. doi: 10.1113/jphysiol.1983.sp014844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Johannisson T., Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981 Jan;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. On the multiplicity of neuronal pathways from muscle spindles and tendon organs and their selection in the cat. J Physiol (Paris) 1982;78(8):772–774. [PubMed] [Google Scholar]
- Lüscher H. R., Mathis J., Henneman E. Wiring diagrams of functional connectivity in monosynaptic reflex arcs of the spinal cord. Neurosci Lett. 1984 Mar 23;45(2):217–222. doi: 10.1016/0304-3940(84)90102-2. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Topographic distribution of terminals of Ia and group II fibers in spinal cord, as revealed by postsynaptic population potentials. J Neurophysiol. 1980 Apr;43(4):968–985. doi: 10.1152/jn.1980.43.4.968. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]


