Abstract
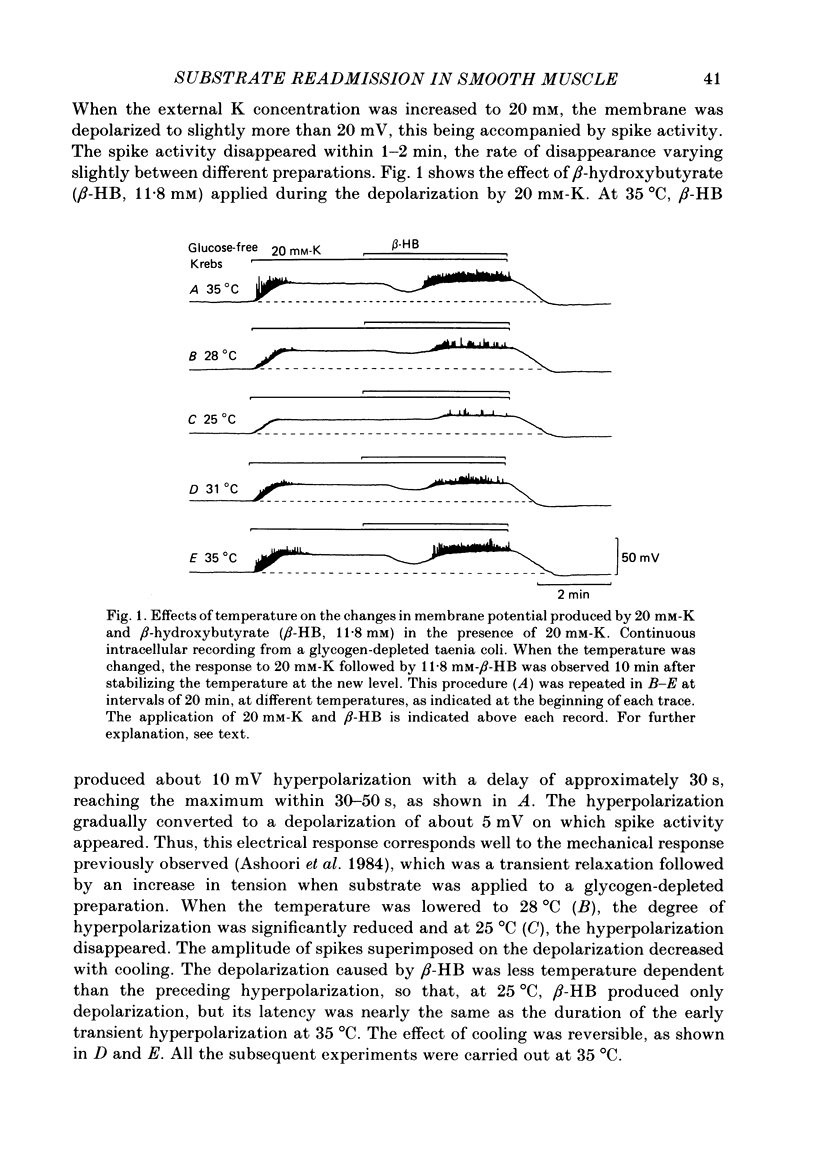
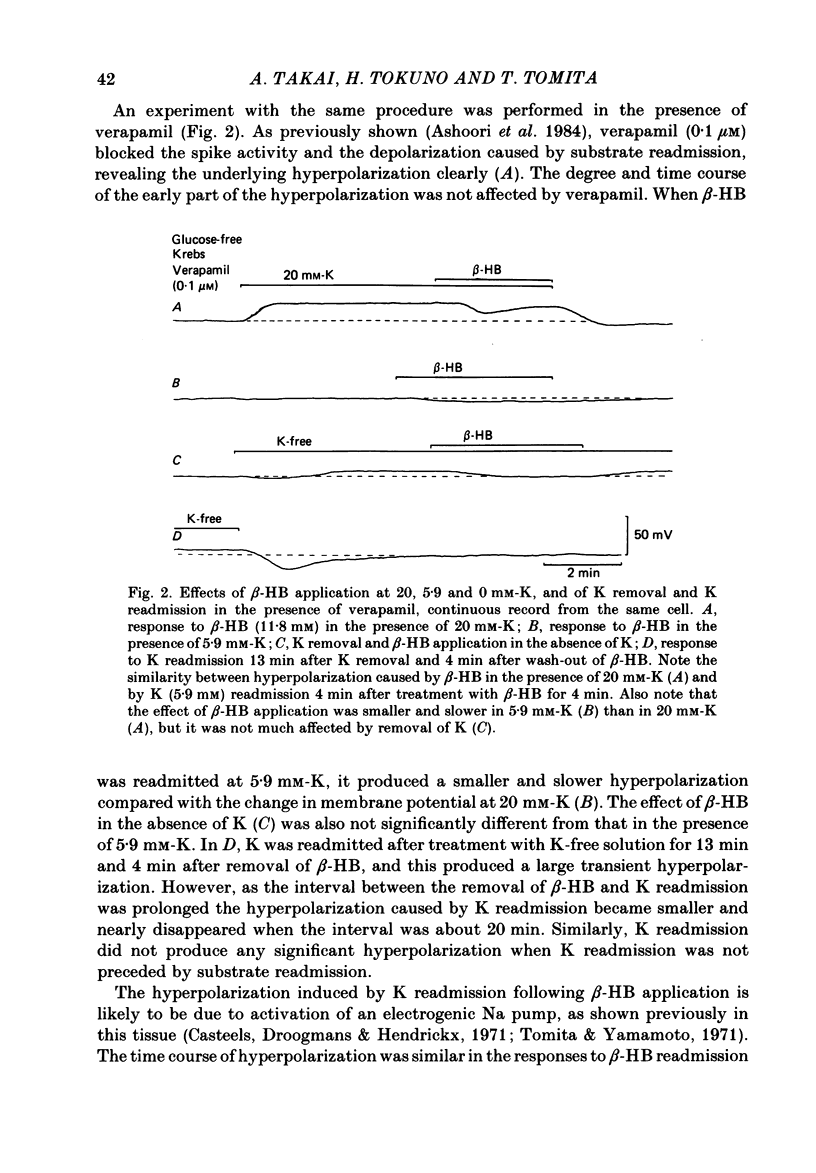
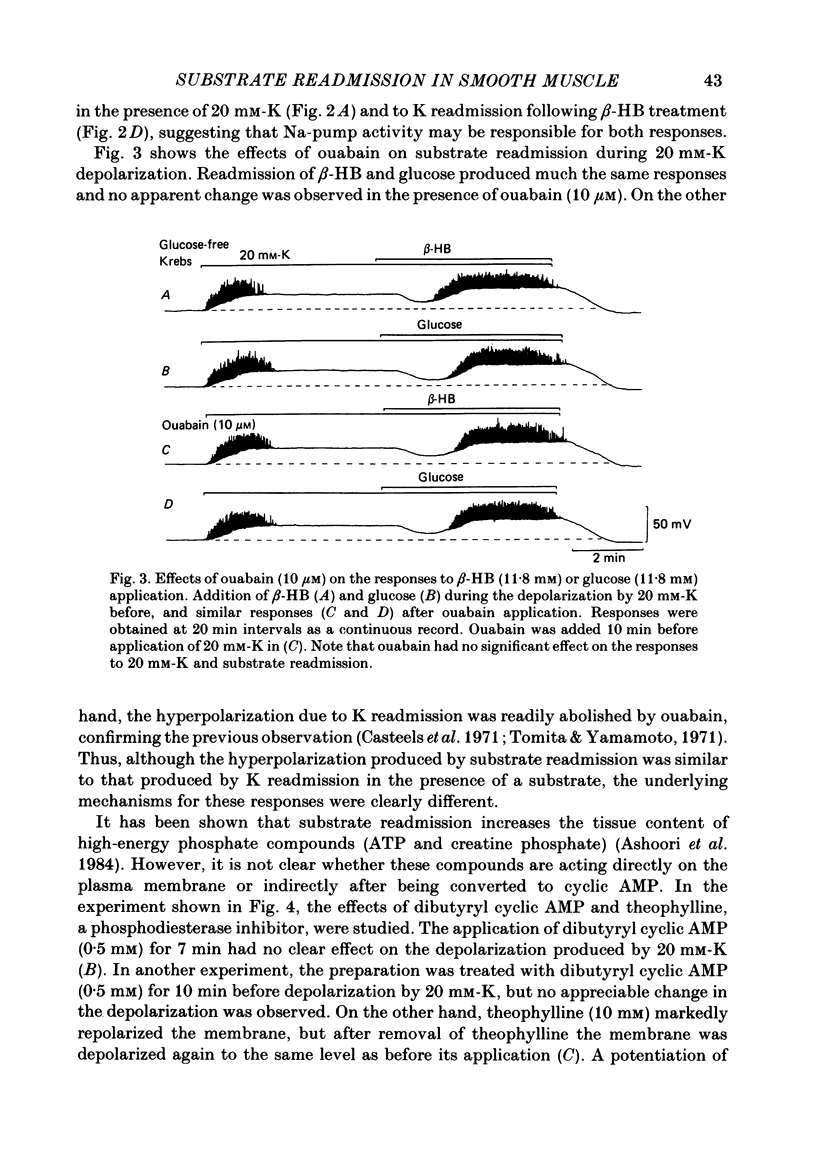
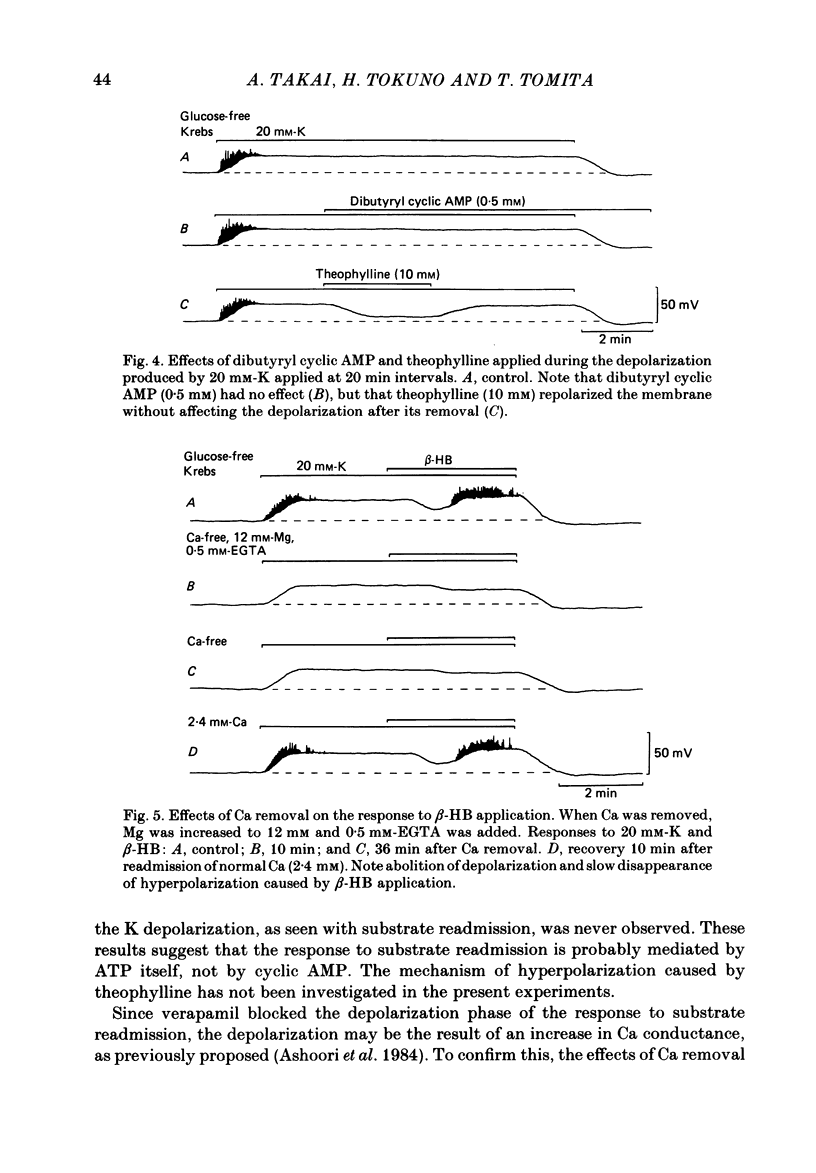
In the glycogen-depleted smooth muscle of the guinea-pig taenia coli, application of glucose or beta-hydroxybutyrate (beta-HB) in the presence of 20 mM-K or carbachol (5 microM) produced a transient hyperpolarization for about 1 min followed by a sustained depolarization accompanied by spike activity. The early hyperpolarization was highly temperature dependent, so that below 30 degrees C, substrate application produced the depolarization with a delay of approximately 2 min, without a clear preceding hyperpolarization. The responses to substrate were not affected by ouabain (10 microM). Readmission of K after a treatment with K-free solution for 10 min caused a transient hyperpolarization only in the presence of substrate. This hyperpolarization was abolished by ouabain. Verapamil (0.1 microM) blocked the substrate-induced depolarization, revealing an underlying slow hyperpolarization. Removal of Ca abolished both the hyperpolarization and depolarization caused by substrate application, but the hyperpolarization disappeared much more slowly than the depolarization. Removal of the external K had little effect on the substrate-induced hyperpolarization, but the hyperpolarization induced by substrate application was increased when the external K was increased up to approximately 20 mM. Both the hyperpolarization and the depolarization were not clearly affected by completely replacing Na with choline. The results suggest that ATP supplied by the addition of substrate activates some electrogenic pump, probably a Ca pump, causing hyperpolarization, and that ATP also removes the inactivation of Ca conductance with some delay, resulting in an increased depolarization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., BULBRING E. Metabolic factors affecting the electrical activity of intestinal smooth muscle. J Physiol. 1961 Apr;156:344–356. doi: 10.1113/jphysiol.1961.sp006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELSSON J., HOEGBERG S. G., TIMMS A. R. THE EFFECT OF REMOVING AND READMITTING GLUCOSE ON THE ELECTRICAL AND MECHANICAL ACTIVITY AND GLUCOSE AND GLYCOGEN CONTENT OF INTESTINAL SMOOTH MUSCLE FROM THE TAENIA COLI OF THE GUINEA PIG. Acta Physiol Scand. 1965 May-Jun;64:28–42. doi: 10.1111/j.1748-1716.1965.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Ashoori F., Takai A., Tokuno H., Tomita T. Effects of glucose removal and readmission on potassium contracture in the guinea-pig taenia coli. J Physiol. 1984 Nov;356:33–48. doi: 10.1113/jphysiol.1984.sp015451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUEDING E., HAWKINS J. T. ENZYMIC DEGRADATION AND MICRODETERMINATION OF GLYCOGEN. Anal Biochem. 1964 Jan;7:26–36. doi: 10.1016/0003-2697(64)90116-2. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Electrogenic sodium pump in smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Sep;217(2):297–313. doi: 10.1113/jphysiol.1971.sp009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. The calcium pump and sodium-calcium exchange in squid axons. Annu Rev Physiol. 1983;45:313–324. doi: 10.1146/annurev.ph.45.030183.001525. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]


