Abstract
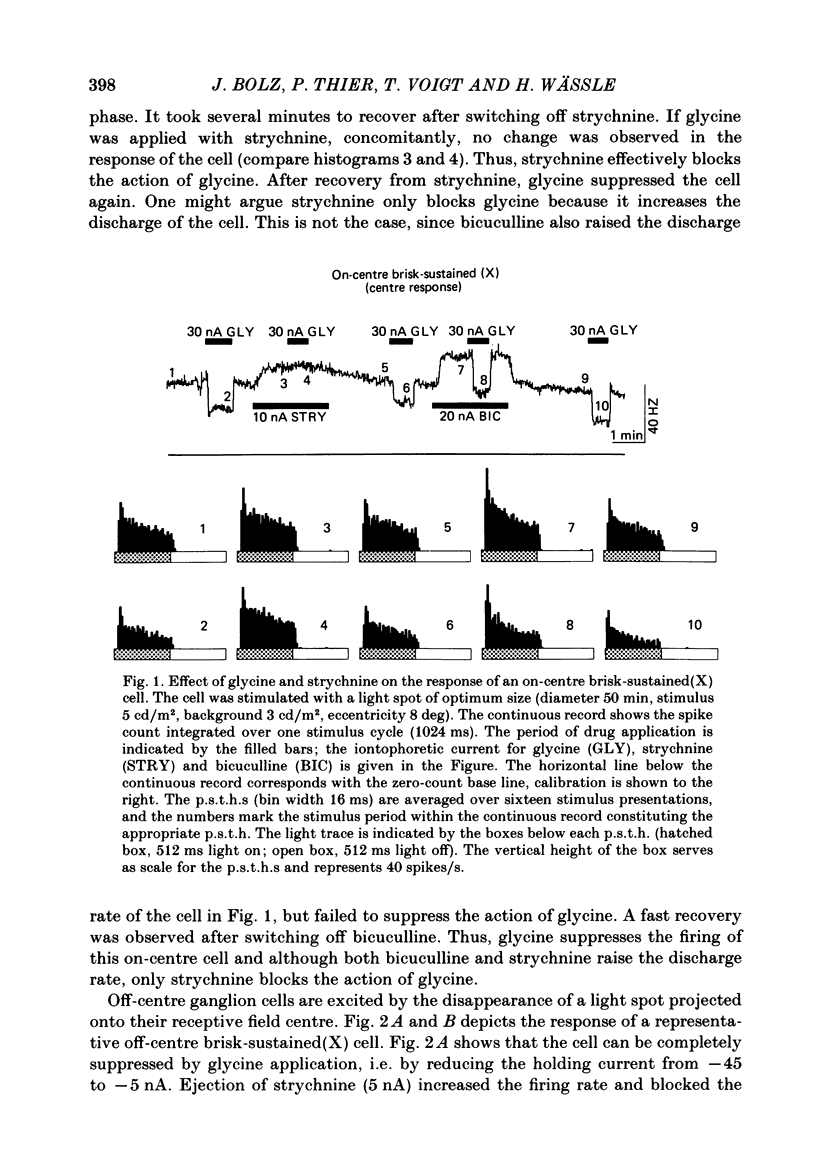
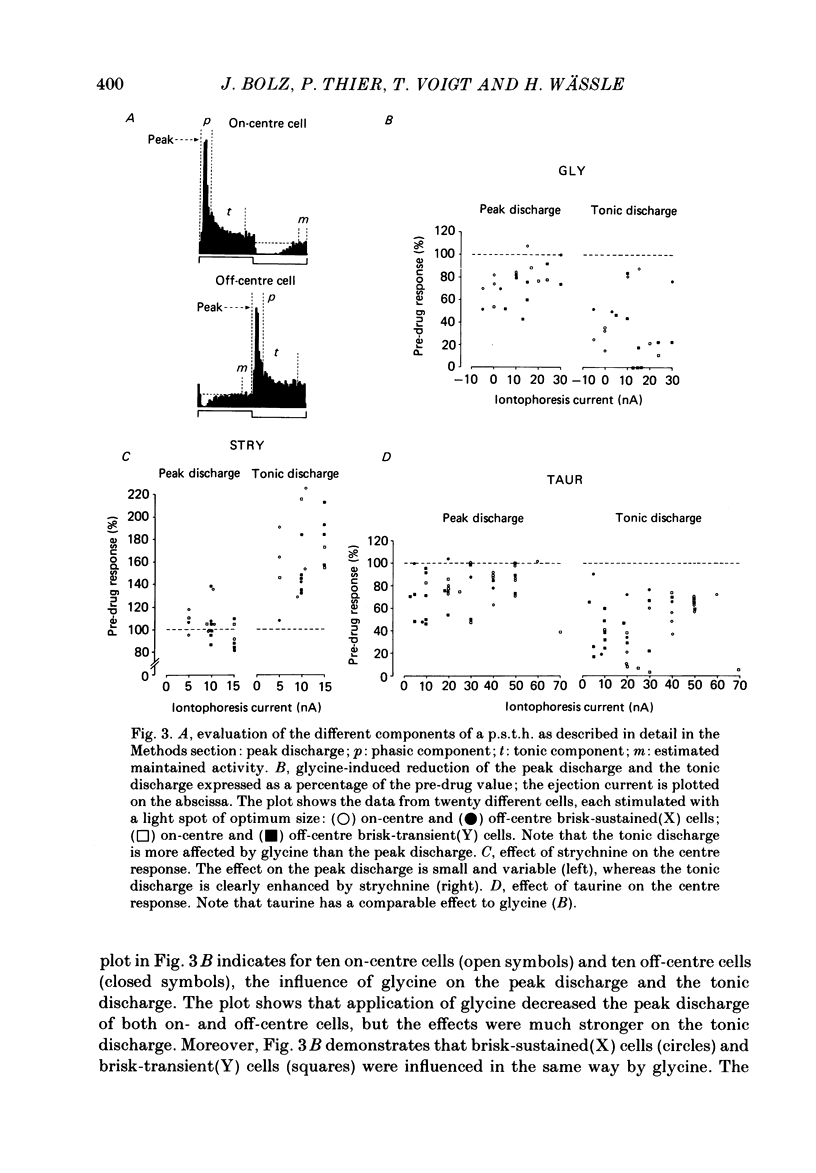
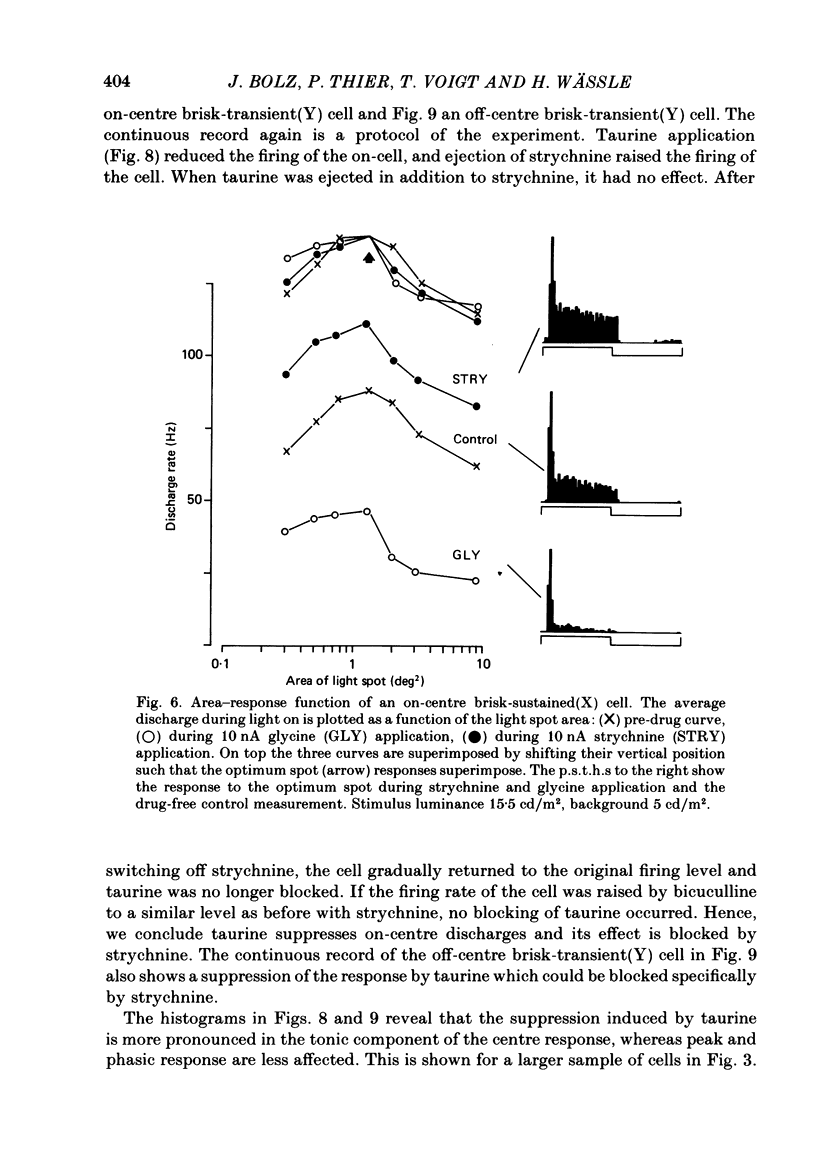
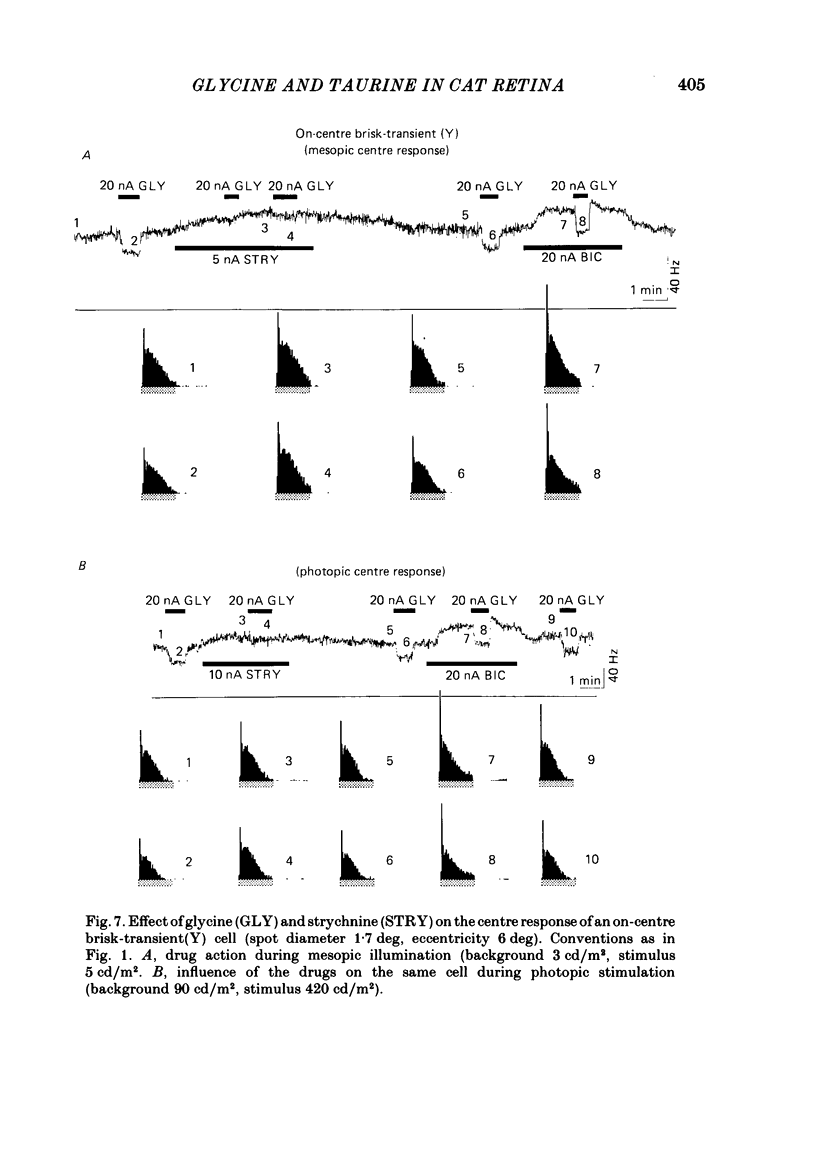
The effects on retinal ganglion cells of iontophoretically applied glycine, taurine and strychnine were studied in the optically intact eye of the cat. Glycine and taurine suppressed the light-evoked discharge of all on-centre and off-centre brisk ganglion cells, regardless of the visual stimulus used. Strychnine blocked the action of externally applied glycine and taurine. The light-evoked response of all ganglion cells was raised by strychnine. The tonic discharge of the light response was suppressed or raised by the drugs more than the phasic response. A population of amacrine cells, which was heavily labelled by [3H]glycine, did not take up [3H]taurine. [3H]taurine was only weakly accumulated by inner nuclear layer neurones and was predominantly located in the outer retina.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Matthews R. The action of light on the eye: Part III. The interaction of retinal neurones. J Physiol. 1928 Jun 24;65(3):273–298. doi: 10.1113/jphysiol.1928.sp002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Frumkes T., Voigt T., Wässle H. Action and localization of gamma-aminobutyric acid in the cat retina. J Physiol. 1985 May;362:369–393. doi: 10.1113/jphysiol.1985.sp015684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure N., Wioland N., Mandel P. Antagonists of the putative inhibitory transmitter effects of taurine and GABA in the retina. Brain Res. 1974 Nov 15;80(2):281–289. doi: 10.1016/0006-8993(74)90691-x. [DOI] [PubMed] [Google Scholar]
- Bonaventure N., Wioland N., Roussel G. Effects of some amino acids (GABA, glycine, taurine) and of their antagonists (picrotoxin, strychnine) on spatial and temporal features of frog retinal ganglion cell responses. Pflugers Arch. 1980 May;385(1):51–64. doi: 10.1007/BF00583915. [DOI] [PubMed] [Google Scholar]
- Borbe H. O., Müller W. E., Wollert U. Specific [3H]strychnine binding associated with glycine receptors in bovine retina. Brain Res. 1981 Jan 26;205(1):131–139. doi: 10.1016/0006-8993(81)90725-3. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. J Comp Neurol. 1973 Mar 1;148(1):91–114. doi: 10.1002/cne.901480106. [DOI] [PubMed] [Google Scholar]
- Bruun A., Ehinger B. Uptake of the putative neurotransmitter, glycine, into the rabbit retina. Invest Ophthalmol. 1972 Apr;11(4):191–198. [PubMed] [Google Scholar]
- Chin C. A., Lam D. M. The uptake and release of [3H]glycine in the goldfish retina. J Physiol. 1980 Nov;308:185–195. doi: 10.1113/jphysiol.1980.sp013467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. Strychnine-sensitive and -insensitive inhibitions in the cat's retina. Tohoku J Exp Med. 1968 Sep;96(1):37–43. doi: 10.1620/tjem.96.37. [DOI] [PubMed] [Google Scholar]
- Cohen A. I., McDaniel M., Orr H. Absolute levels of some free amino acids in normal and biologically fractionated retinas. Invest Ophthalmol. 1973 Sep;12(9):686–693. [PubMed] [Google Scholar]
- Cunningham R. A., Miller R. F. Electrophysiological analysis of taurine and glycine action on neurons of the mudpuppy retina. II. ERG, PNR and Müller cell recordings. Brain Res. 1980 Sep 15;197(1):139–151. doi: 10.1016/0006-8993(80)90440-0. [DOI] [PubMed] [Google Scholar]
- Cunningham R., Miller R. F. Electrophysiological analysis of taurine and glycine action on neurons of the midpuppy retina. I. Intracellular recording. Brain Res. 1980 Sep 15;197(1):123–138. doi: 10.1016/0006-8993(80)90439-4. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res. 1971 Jun 29;12(5):547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- Di Giorgio R. M., Macaione S., De Luca G. Subcellular distribution of hypotaurine oxidase activity in ox retina. Life Sci. 1977 May 15;20(10):1657–1662. doi: 10.1016/0024-3205(77)90339-3. [DOI] [PubMed] [Google Scholar]
- Di Giorgio R. M., Tucci G., Macaione S. Cysteine oxidase activity in rat retina during development. Life Sci. 1975 Feb 1;16(3):429–436. doi: 10.1016/0024-3205(75)90264-7. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Cells accumulating 3h-glycine in the goldfish retina. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;217(1):1–7. doi: 10.1007/BF00410875. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971 Oct 8;33(1):157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Lindberg-Bauer B. Light-evoked release of glycine from cat and rabbit retina. Brain Res. 1976 Sep 3;113(3):535–549. doi: 10.1016/0006-8993(76)90055-x. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Lindberg B. Light-evoked release of glycine from the retina. Nature. 1974 Oct 25;251(5477):727–728. doi: 10.1038/251727a0. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976 Oct 8;194(4261):193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Hösli L. Strychnine and inhibition of bulbar reticular neurones. Experientia. 1973 May 15;29(5):542–544. doi: 10.1007/BF01926653. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res. 1973 Mar 30;52:399–402. doi: 10.1016/0006-8993(73)90680-x. [DOI] [PubMed] [Google Scholar]
- Hayes K. C., Carey R. E., Schmidt S. Y. Retinal degeneration associated with taurine deficiency in the cat. Science. 1975 May 30;188(4191):949–951. doi: 10.1126/science.1138364. [DOI] [PubMed] [Google Scholar]
- Heiss W. D., Bornschein H. Die Impulsverteilung der Daueraktivität von Einzelfasern des N. opticus. Einflüsse von Licht, Ischämie, Strychnin und Barbiturat. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Sep 21;286(1):1–18. [PubMed] [Google Scholar]
- Heiss W. D. Daueraktivität retinaler Neurone unter Einwirkung von Strychnin und Pikrotoxin. Vision Res. 1967 Jul;7(7):583–598. doi: 10.1016/0042-6989(67)90067-3. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Sheardown M. J. Transmitters mediating inhibition of ganglion cells in the cat retina: iontophoretic studies in vivo. Neuroscience. 1983 Apr;8(4):837–853. doi: 10.1016/0306-4522(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Kennedy A. J., Neal M. J., Lolley R. N. The distribution of amino acids within the rat retina. J Neurochem. 1977 Jul;29(1):157–159. doi: 10.1111/j.1471-4159.1977.tb03938.x. [DOI] [PubMed] [Google Scholar]
- Kennedy A. J., Neal M. J. The effect of light and potassium depolarization on the release of endogenous amino acids from the isolated rat retina. Exp Eye Res. 1978 Jan;26(1):71–75. doi: 10.1016/0014-4835(78)90153-7. [DOI] [PubMed] [Google Scholar]
- Kirby A. W. The effect of strychnine, bicuculline, and picrotoxin on X and Y cells in the cat retina. J Gen Physiol. 1979 Jul;74(1):71–84. doi: 10.1085/jgp.74.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake N., Marshall J., Voaden M. J. High affinity uptake sites for taurine in the retina. Exp Eye Res. 1978 Dec;27(6):713–8. doi: 10.1016/0014-4835(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Lam D. M. Glycinergic pathways in the goldfish retina. J Neurosci. 1981 Feb;1(2):152–165. doi: 10.1523/JNEUROSCI.01-02-00152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J., Voaden M. An investigation of the cells incorporating (3H)GABA and (3H)glycine in the isolated retina of the rat. Exp Eye Res. 1974 Apr;18(4):367–370. doi: 10.1016/0014-4835(74)90113-4. [DOI] [PubMed] [Google Scholar]
- Mathur R. L., Klethi J., Ledig M., Mandel P. Cysteine sulfinate carboxylase in the visual pathway of adult chicken. Life Sci. 1976 Jan 1;18(1):75–79. doi: 10.1016/0024-3205(76)90276-9. [DOI] [PubMed] [Google Scholar]
- Nelson R. AII amacrine cells quicken time course of rod signals in the cat retina. J Neurophysiol. 1982 May;47(5):928–947. doi: 10.1152/jn.1982.47.5.928. [DOI] [PubMed] [Google Scholar]
- Nishimura C., Ida S., Kuriyama K. Taurine biosynthesis in frog retina: effects of light and dark adaptations. J Neurosci Res. 1983;9(1):59–67. doi: 10.1002/jnr.490090108. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Cohen A. I., Lowry O. H. The distribution of taurine in the vertebrate retina. J Neurochem. 1976 Mar;26(3):609–611. doi: 10.1111/j.1471-4159.1976.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Klethi J., Ledig M., Mandel P. Free amino acids of chicken and rat retina. Brain Res. 1972 Jun 22;41(2):494–497. doi: 10.1016/0006-8993(72)90523-9. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G. [3H]taurine-accumulating neurons in the cat retina. Exp Eye Res. 1981 Jan;32(1):11–20. doi: 10.1016/s0014-4835(81)80034-6. [DOI] [PubMed] [Google Scholar]
- Rayborn M. E., Sarthy P. V., Lam D. M., Hollyfield J. G. The emergence, localization, and maturation of neurotransmitter systems during development of the retina in Xenopus laevis: II. Glycine. J Comp Neurol. 1981 Feb 1;195(4):585–593. doi: 10.1002/cne.901950404. [DOI] [PubMed] [Google Scholar]
- Saito H. The effects of strychnine and bicuculline on the responses of X- and Y-cells of the isolated eye-cut preparation of the cat. Brain Res. 1981 May 11;212(1):243–248. doi: 10.1016/0006-8993(81)90061-5. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Starr M. S. Effect of dark adaptation on the GABA system in retina. Brain Res. 1973 Sep 14;59:331–338. doi: 10.1016/0006-8993(73)90271-0. [DOI] [PubMed] [Google Scholar]
- Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- Urban P. F., Dreyfus H., Mandel P. Influence of various amino acids on the bioelectrical response to light stimulation of a superfused frog retina. Life Sci. 1976 Mar 1;18(5):473–479. doi: 10.1016/0024-3205(76)90324-6. [DOI] [PubMed] [Google Scholar]
- Voaden M. J., Lake N., Marshall J., Morjaria B. Studies on the distribution of taurine and other neuroactive amino acids in the retina. Exp Eye Res. 1977 Sep;25(3):249–257. doi: 10.1016/0014-4835(77)90091-4. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wässle H., Boycott B. B., Illing R. B. Morphology and mosaic of on- and off-beta cells in the cat retina and some functional considerations. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):177–195. doi: 10.1098/rspb.1981.0033. [DOI] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Morphology and topography of on- and off-alpha cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]
- Yates R. A., Keen P. The distribution of free amino acids in subdivision of rat and frog retinae obtained by a new technique. Brain Res. 1976 Apr 30;107(1):117–126. doi: 10.1016/0006-8993(76)90099-8. [DOI] [PubMed] [Google Scholar]



