Abstract
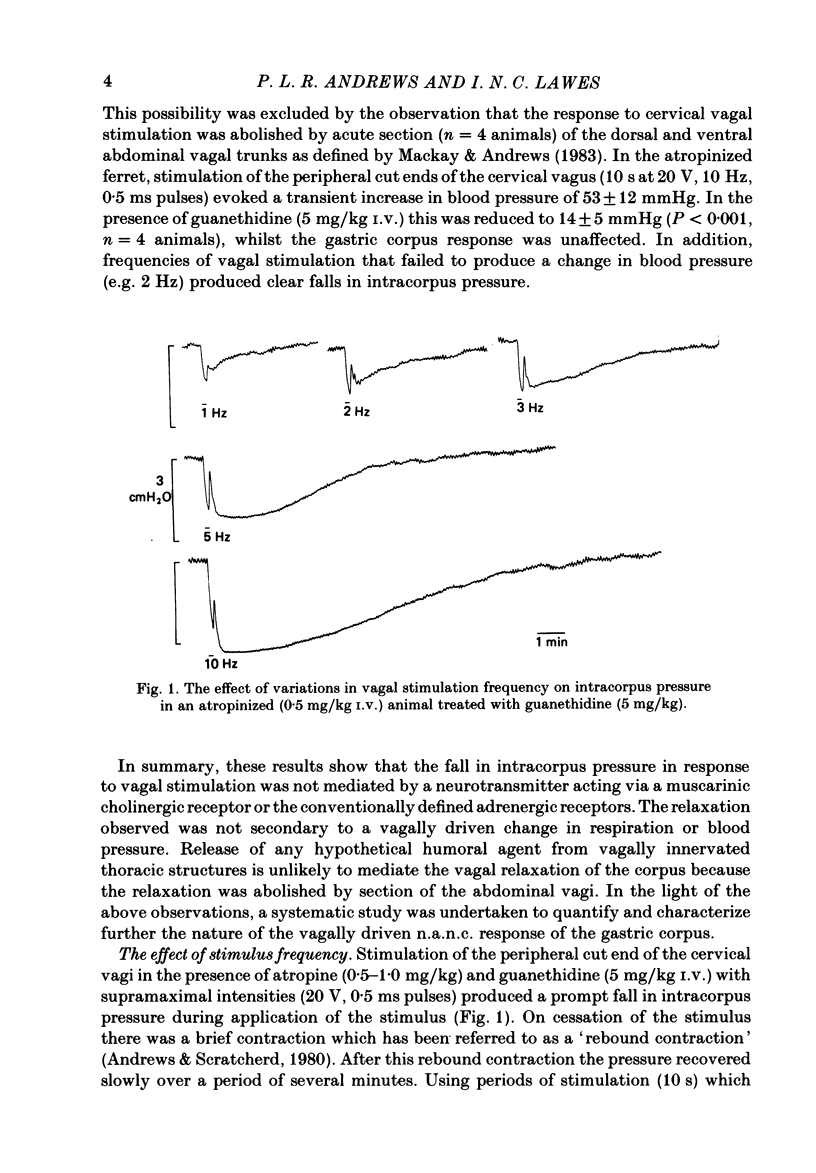
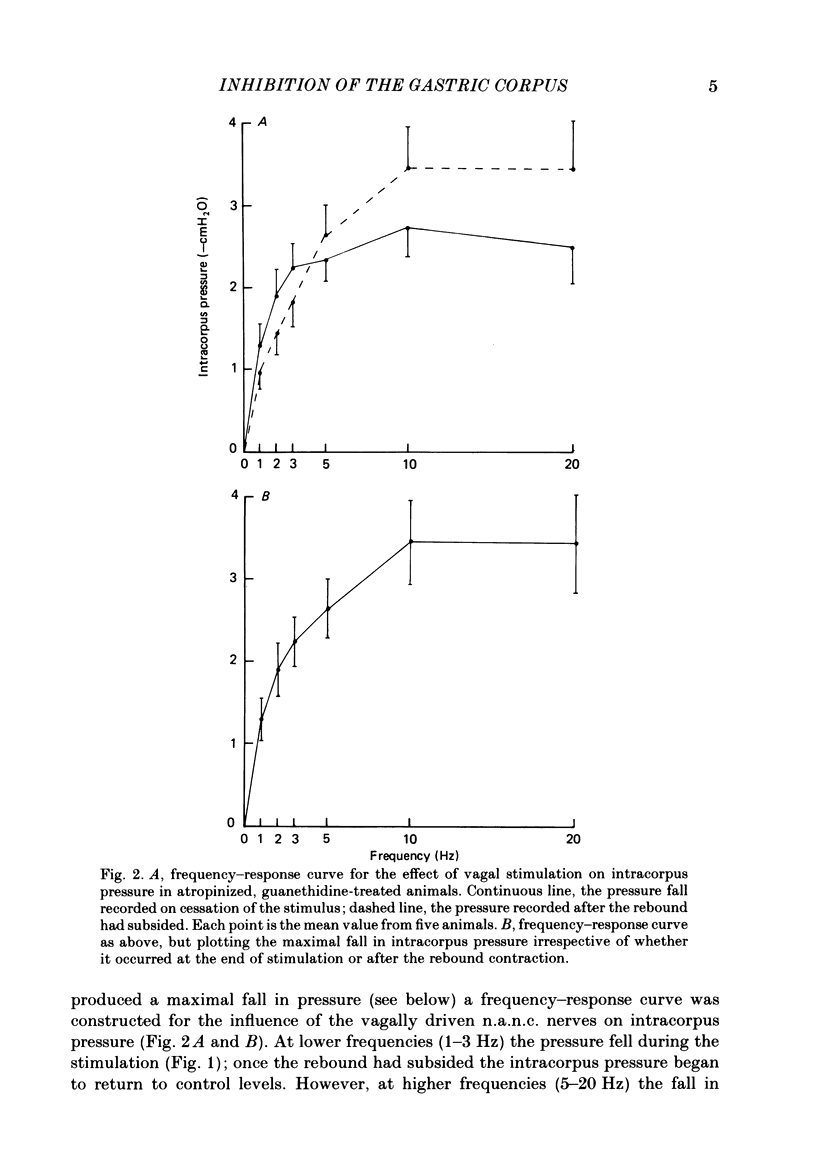
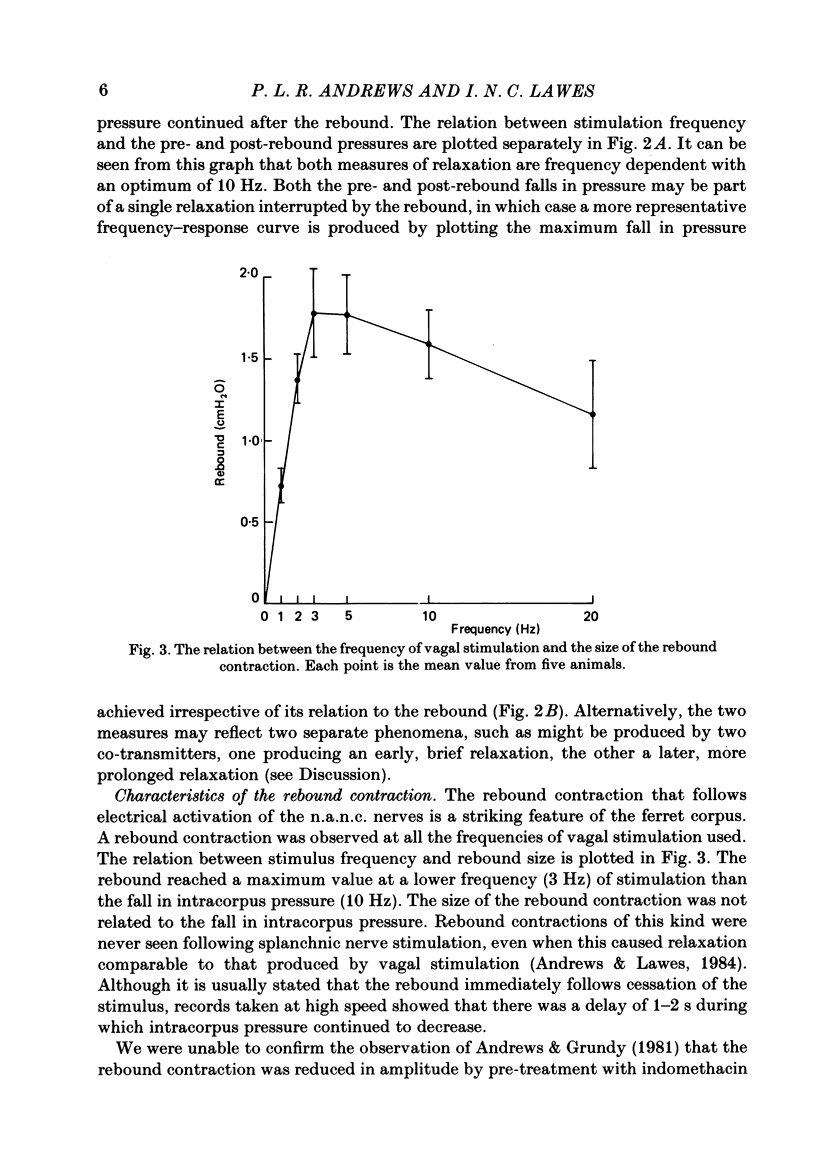
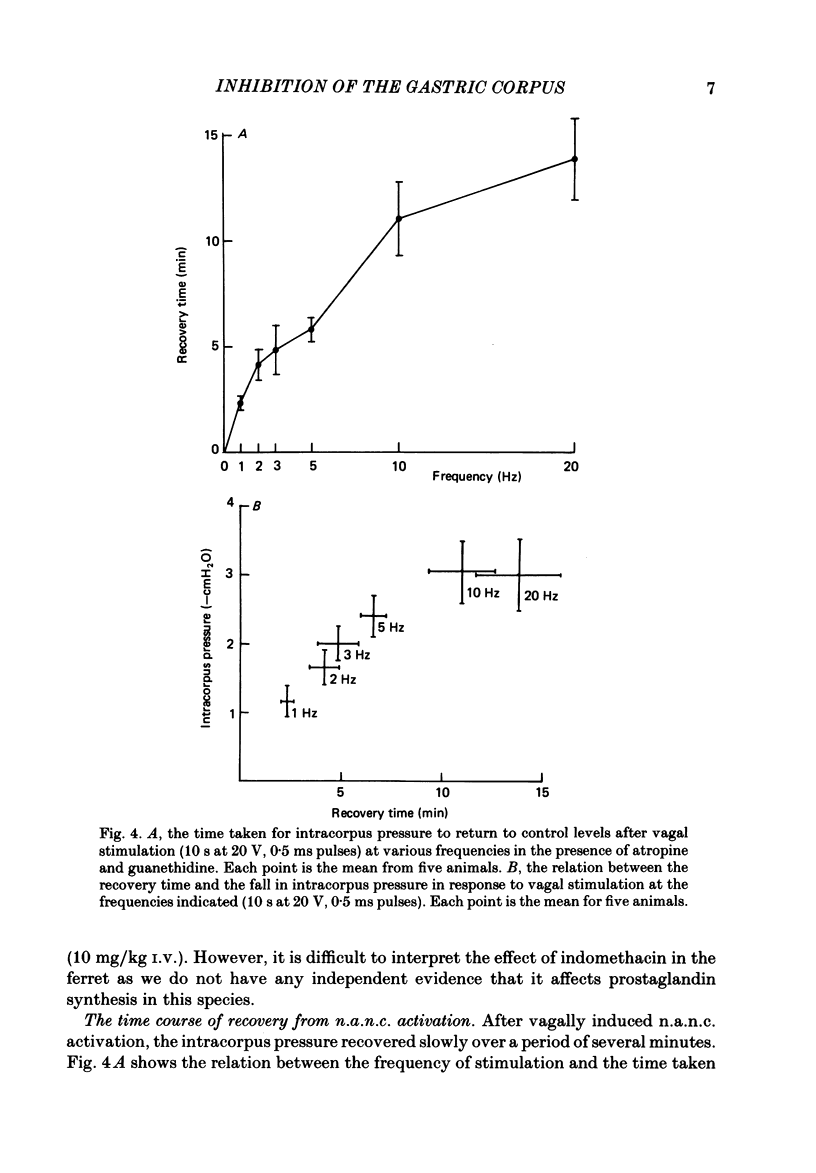
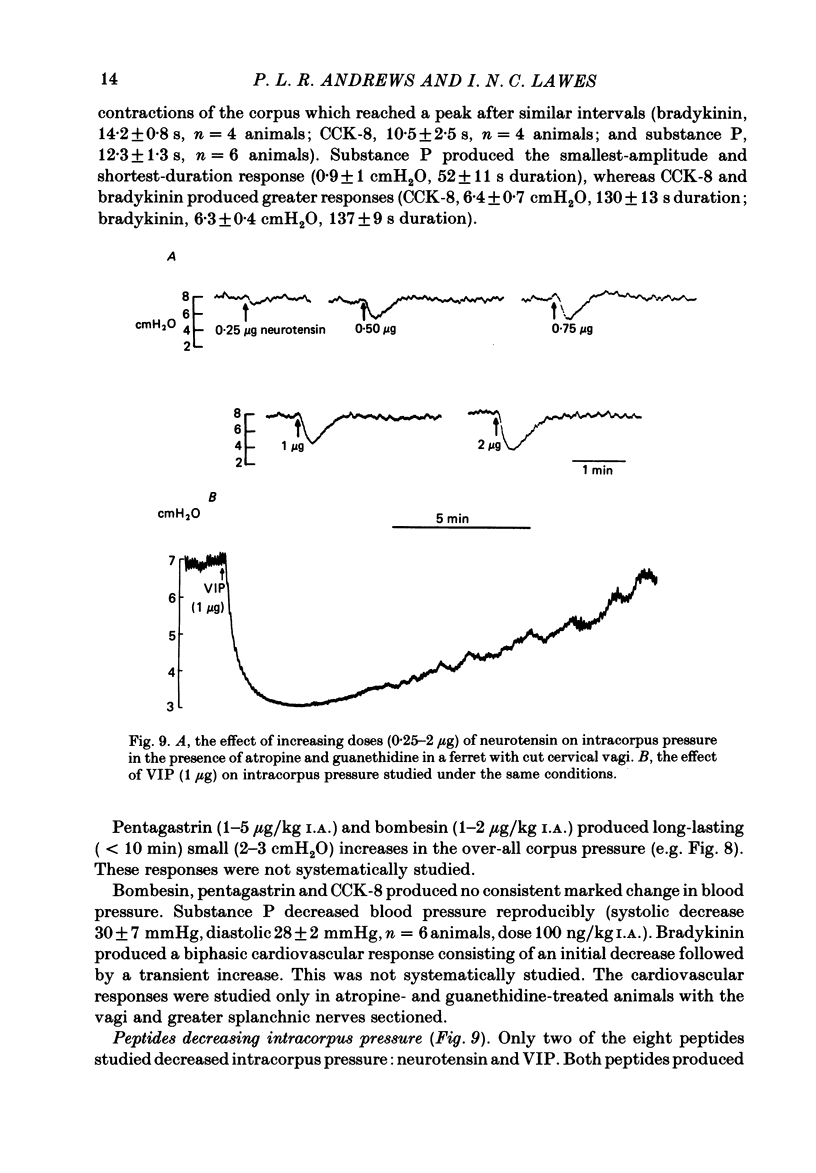
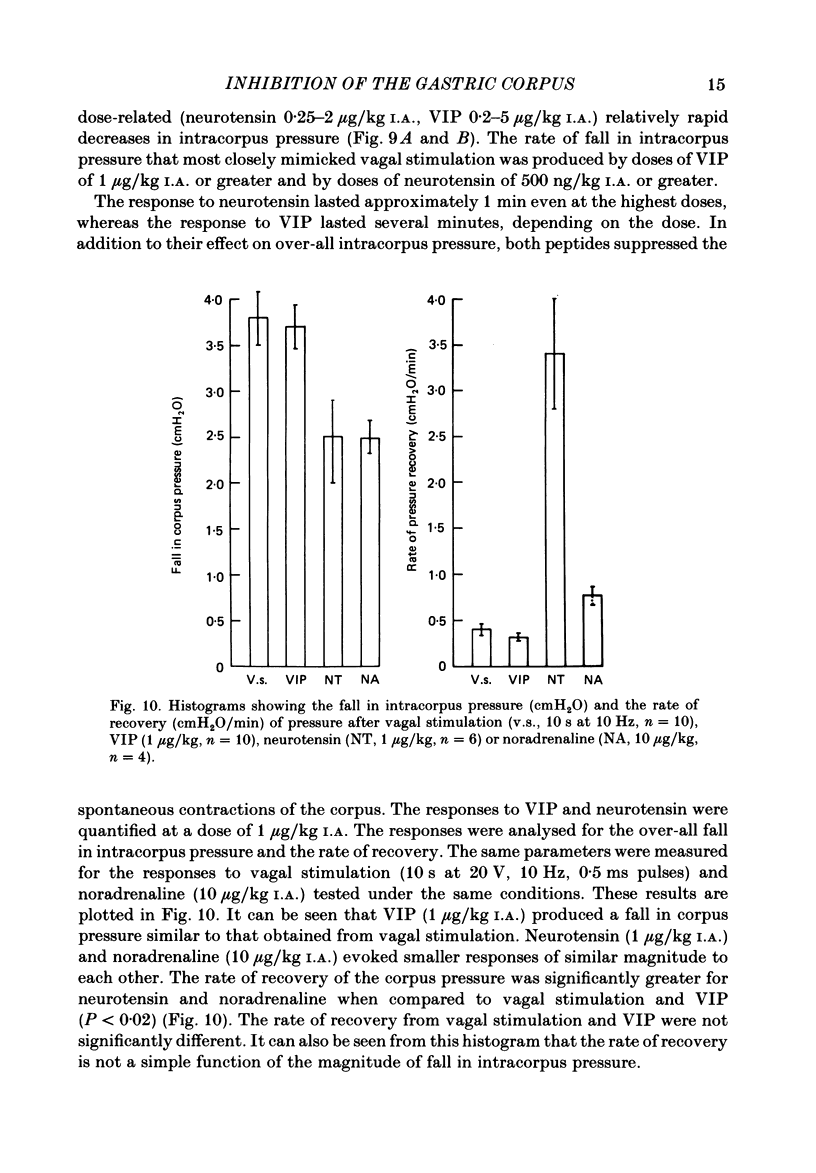
This paper reports a quantitative in vivo study on the vagal activation of the intramural non-adrenergic, non-cholinergic inhibitory nerves in the ferret gastric corpus. The nature of the inhibitory neurotransmitter was also investigated. In the atropinized, guanethidine-treated, urethane-anaesthetized ferret, electrical stimulation (10 s at 20 V, 1-20 Hz, 0.5 ms pulses) of the cervical vagi produced a prompt fall in intracorpus pressure that was related to the stimulus frequency. The maximal response was achieved at 10 Hz. The time taken for the intracorpus pressure to return to pre-stimulus levels after a 10 s period of stimulation was related to the stimulus frequency; at 10 Hz the pressure took approximately 11 min to recover. In contrast to studies in the cat (Martinson & Muren, 1963), there was no detectable difference in the electrical threshold for activation of the vagal excitatory and vagal inhibitory fibres. The nature of the vagal non-adrenergic, non-cholinergic inhibitory neurotransmitter was investigated using a variety of antagonists and agonists. Adenosine triphosphate (ATP), adenosine, alpha beta-methylene ATP and beta gamma-methylene ATP all contracted the corpus in the presence of vagotomy, atropine, guanethidine and indomethacin. The vagally induced fall in corpus pressure was not blocked by high doses of alpha beta-methylene ATP. A variety of peptides were investigated for their effects on corpus pressure in the presence of atropine, guanethidine and vagotomy. Bombesin, pentagastrin, substance P, cholecystokinin octapeptide (CCK-8) and bradykinin all produced an increase in intracorpus pressure. Neurotensin and vasoactive intestinal polypeptide (VIP) both decreased intracorpus pressure, and of the two VIP most closely mimicked the response to vagal activation of the non-cholinergic, non-adrenergic inhibitory neurones. The results provide support for the involvement of a peptide (possibly VIP) rather than a purine in the vagally driven decrease in intracorpus pressure in the ferret.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. L., Fussey I. V., Scratcherd T. The spontaneous discharge in abdominal vagal efferents in the dog and ferret. Pflugers Arch. 1980 Aug;387(1):55–60. doi: 10.1007/BF00580844. [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Lawes I. N. Interactions between splanchnic and vagus nerves in the control of mean intragastric pressure in the ferret. J Physiol. 1984 Jun;351:473–490. doi: 10.1113/jphysiol.1984.sp015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. L., Lawes I. N. The role of vagal and intramural inhibitory reflexes in the regulation of intragastric pressure in the ferret. J Physiol. 1982 May;326:435–451. doi: 10.1113/jphysiol.1982.sp014204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. L., Scratcherd T. The gastric motility patterns induced by direct and reflex excitation of the vagus nerves in the anaesthetized ferret. J Physiol. 1980 May;302:363–378. doi: 10.1113/jphysiol.1980.sp013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beani L., Bianchi C., Crema A. Vagal non-adrenergic inhibition of guinea-pig stomach. J Physiol. 1971 Sep;217(2):259–279. doi: 10.1113/jphysiol.1971.sp009570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Paddle B., Staszewska-Barczak J. Evidence that prostaglandin is responsible for the 'rebound contraction' following stimulation of non-adrenergic, non-cholinergic ('purinergic') inhibitory nerves. Eur J Pharmacol. 1975 Apr;31(2):360–362. doi: 10.1016/0014-2999(75)90060-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Comparative studies of purinergic nerves. J Exp Zool. 1975 Oct;194(1):103–133. doi: 10.1002/jez.1401940108. [DOI] [PubMed] [Google Scholar]
- CASS R., KUNTZMAN R., BRODIE B. B. Norepinephrine depletion as a possible mechanism of action of guanethidine (SU 5864), a new hypotensive agent. Proc Soc Exp Biol Med. 1960 Apr;103:871–872. doi: 10.3181/00379727-103-25702. [DOI] [PubMed] [Google Scholar]
- CHANG C. C., COSTA E., BRODIE B. B. INTERACTION OF GUANETHIDINE WITH ADRENERGIC NEURONS. J Pharmacol Exp Ther. 1965 Mar;147:303–312. [PubMed] [Google Scholar]
- Fahrenkrug J., Galbo H., Holst J. J., Schaffalitzky de Muckadell O. B. Influence of the autonomic nervous system on the release of vasoactive intestinal polypeptide from the porcine gastrointestinal tract. J Physiol. 1978 Jul;280:405–422. doi: 10.1113/jphysiol.1978.sp012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978 Nov;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. Transmission to the longitudinal muscle of the guinea-pig vas deferens: The effect of pretreatment with guanethidine. Br J Pharmacol. 1974 Jan;50(1):63–68. doi: 10.1111/j.1476-5381.1974.tb09593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSON G., MARTINSON J. SOME QUANTITATIVE CONSIDERATIONS ON VAGALLY INDUCED RELAXATION OF GASTRIC SMOOTH MUSCLE IN THE CAT. Acta Physiol Scand. 1965 Mar;63:351–357. doi: 10.1111/j.1748-1716.1965.tb04074.x. [DOI] [PubMed] [Google Scholar]
- Liedberg G., Nielsen K. C., Owman C., Sjöberg N. O. Adrenergic contribution to the abdominal vagus nerves in the cat. Scand J Gastroenterol. 1973;8(2):177–180. [PubMed] [Google Scholar]
- Lundberg J., Ahlman H., Dahlström A., Kewenter J. Catecholamine-containing nerve fibres in the human abdominal vagus. Gastroenterology. 1976 Mar;70(3):472–474. [PubMed] [Google Scholar]
- Mackay T. W., Andrews P. L. A comparative study of the vagal innervation of the stomach in man and the ferret. J Anat. 1983 May;136(Pt 3):449–481. [PMC free article] [PubMed] [Google Scholar]
- Martinson J. The effect of graded vagal stimulation on gastric motility, secretion and blood flow in the cat. Acta Physiol Scand. 1965 Dec;65(4):300–309. doi: 10.1111/j.1748-1716.1965.tb04277.x. [DOI] [PubMed] [Google Scholar]
- Morgan K. G., Muir T. C., Szurszewski J. H. The electrical basis for contraction and relaxation in canine fundal smooth muscle. J Physiol. 1981 Feb;311:475–488. doi: 10.1113/jphysiol.1981.sp013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga A., Nakazato Y., Saito K. Considerations of the efferent nervous mechanism of the vago-vagal reflex relaxation of the stomach in the dog. Jpn J Pharmacol. 1970 Mar;20(1):116–130. doi: 10.1254/jjp.20.116. [DOI] [PubMed] [Google Scholar]
- Ohga A., Taneike T. Dissimilarity between the responses to adenosine triphosphate or its related compounds and non-adrenergic inhibitory nerve stimulation in the longitudinal smooth muscle of pig stomach. Br J Pharmacol. 1977 Jun;60(2):221–231. doi: 10.1111/j.1476-5381.1977.tb07744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D., VANE J. R. Analysis of he responses of the isolated stomach to electrical stimulation and to drugs. J Physiol. 1963 Jan;165:10–46. doi: 10.1113/jphysiol.1963.sp007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalz P. F., Morgan K. G., Szurszewski J. H. Pentagastrin potentiates nonadrenergic inhibitory neuromuscular transmission in orad stomach of the dog. Am J Physiol. 1983 Oct;245(4):G597–G600. doi: 10.1152/ajpgi.1983.245.4.G597. [DOI] [PubMed] [Google Scholar]
- Valenzuela J. E. Dopamine as a possible neurotransmitter in gastric relaxation. Gastroenterology. 1976 Dec;71(6):1019–1022. [PubMed] [Google Scholar]


