Abstract
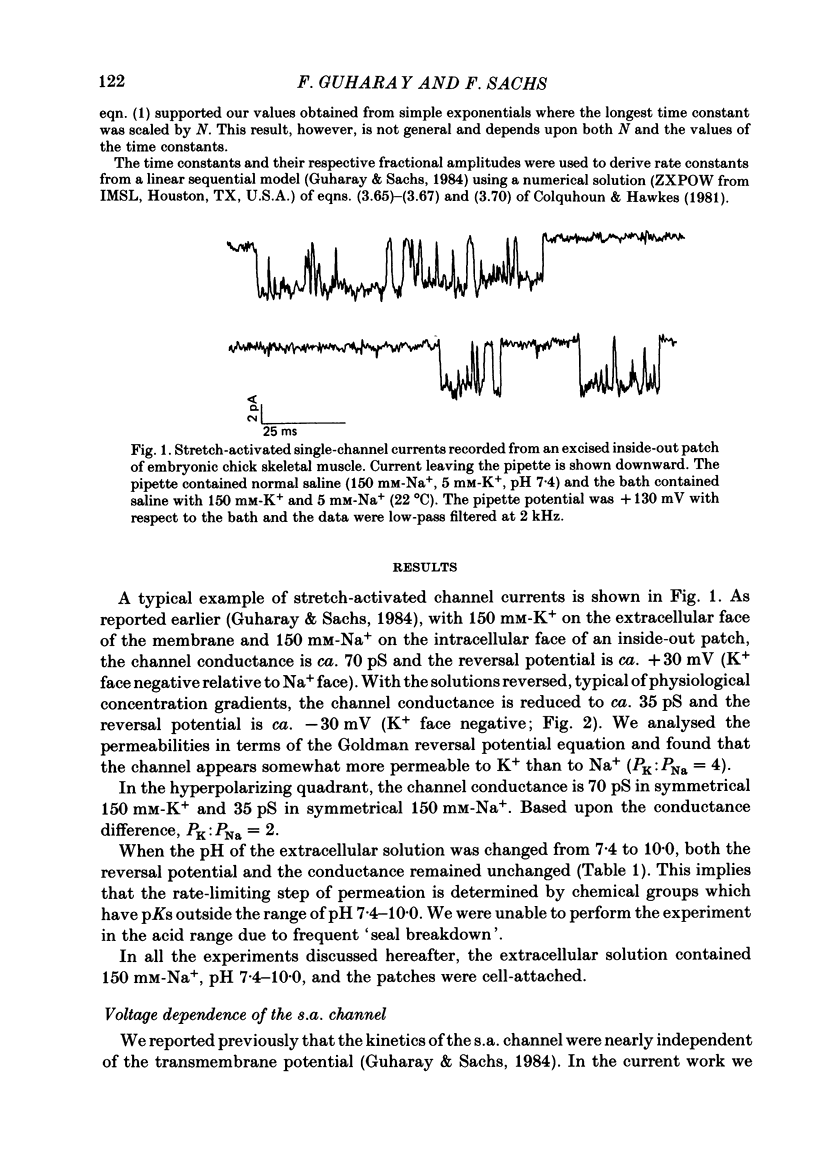
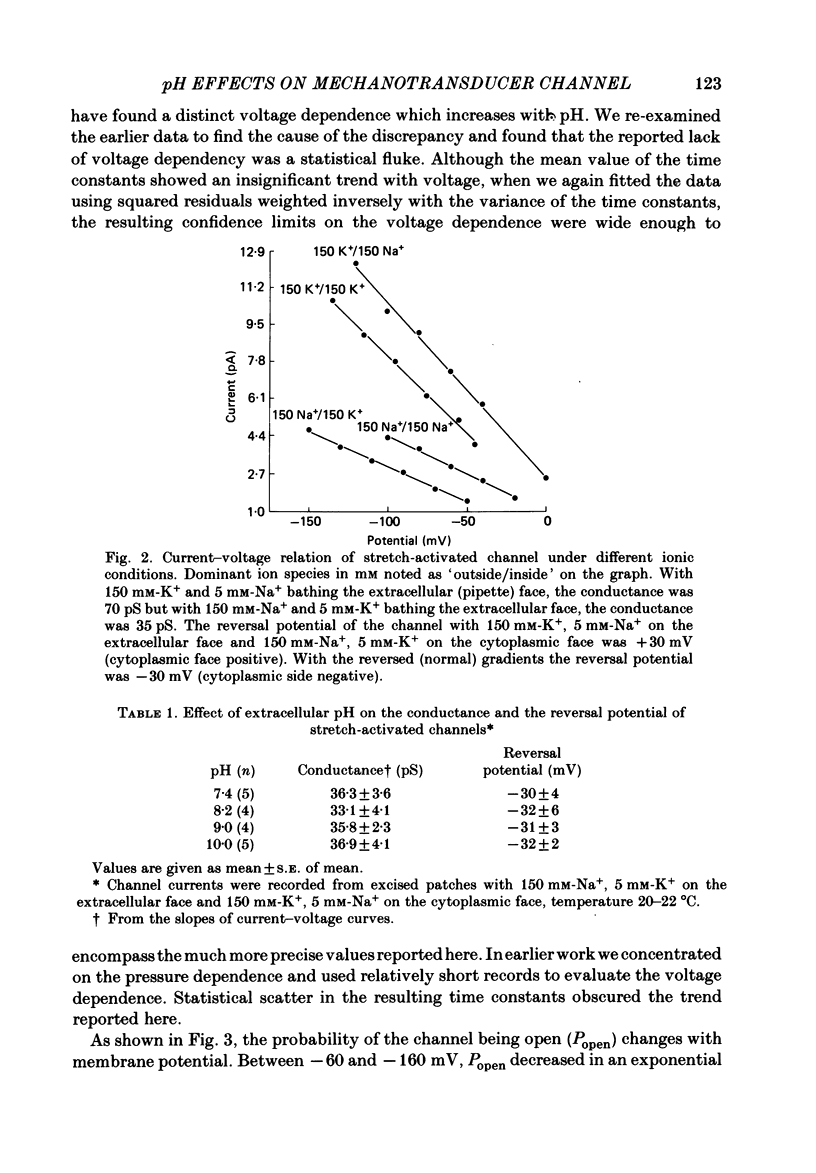
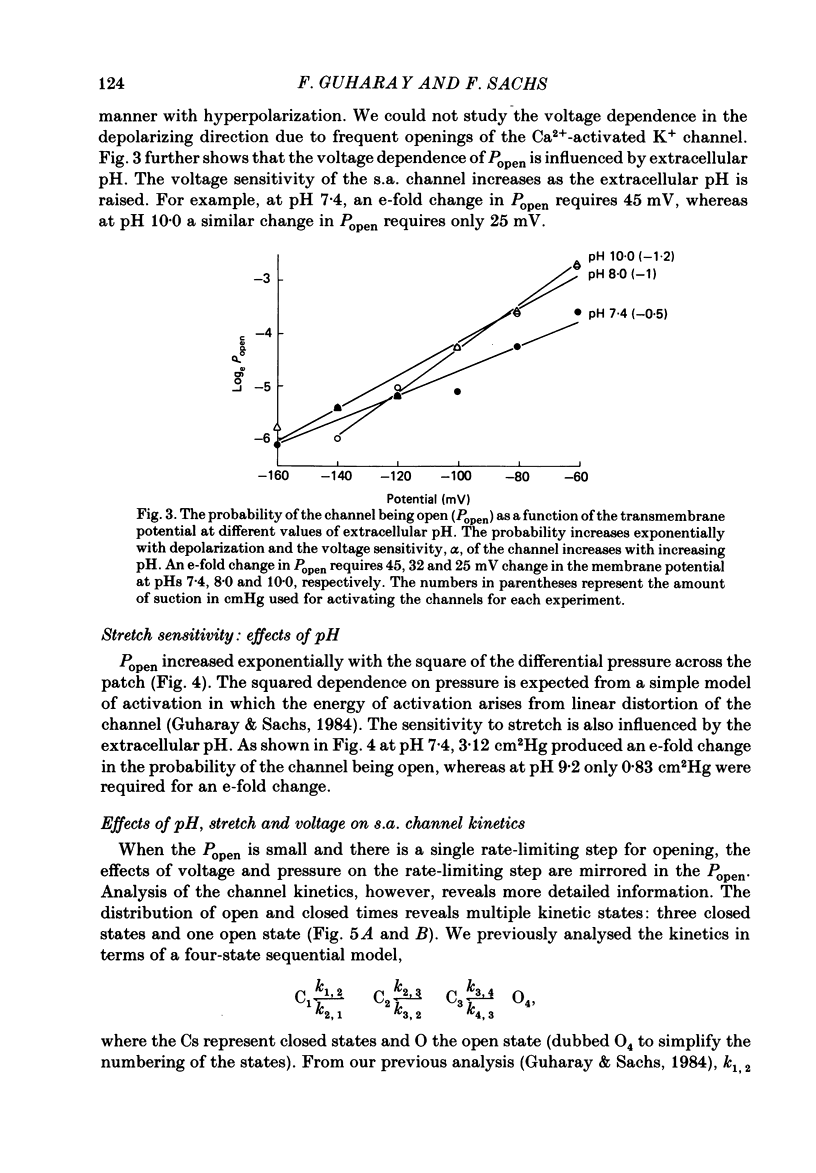
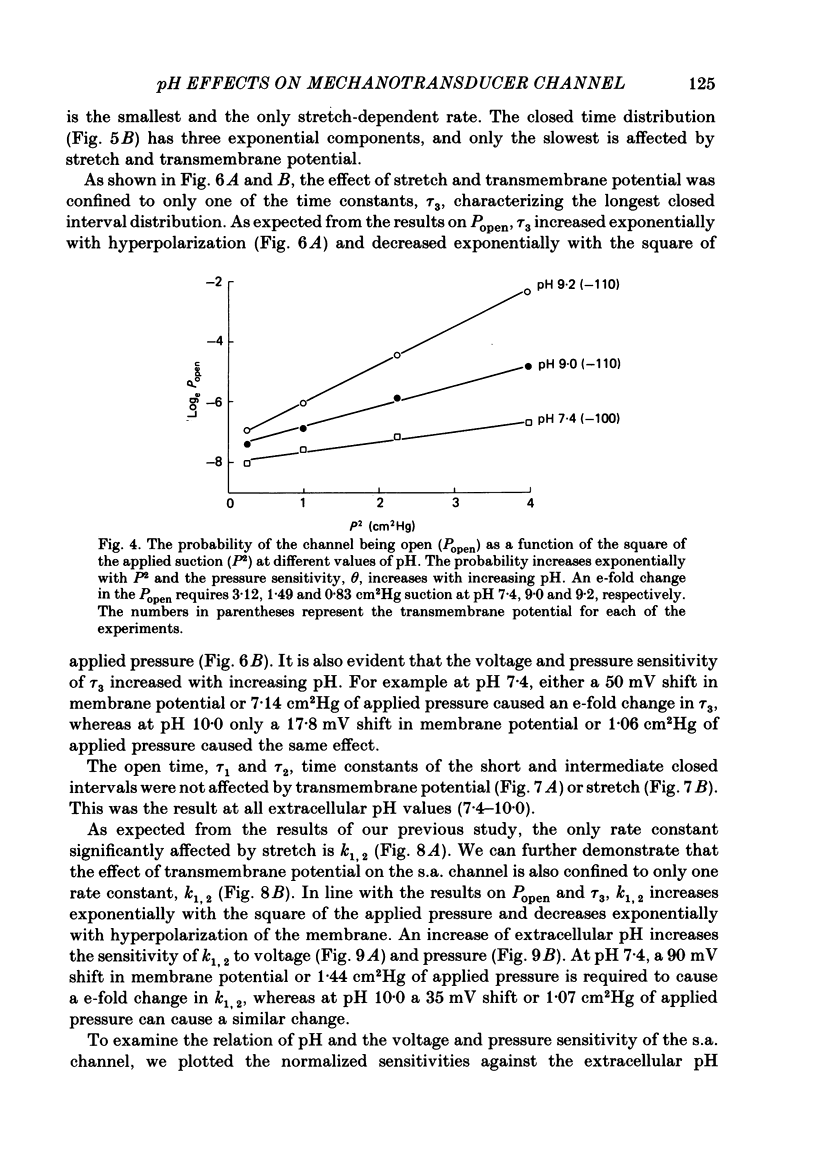
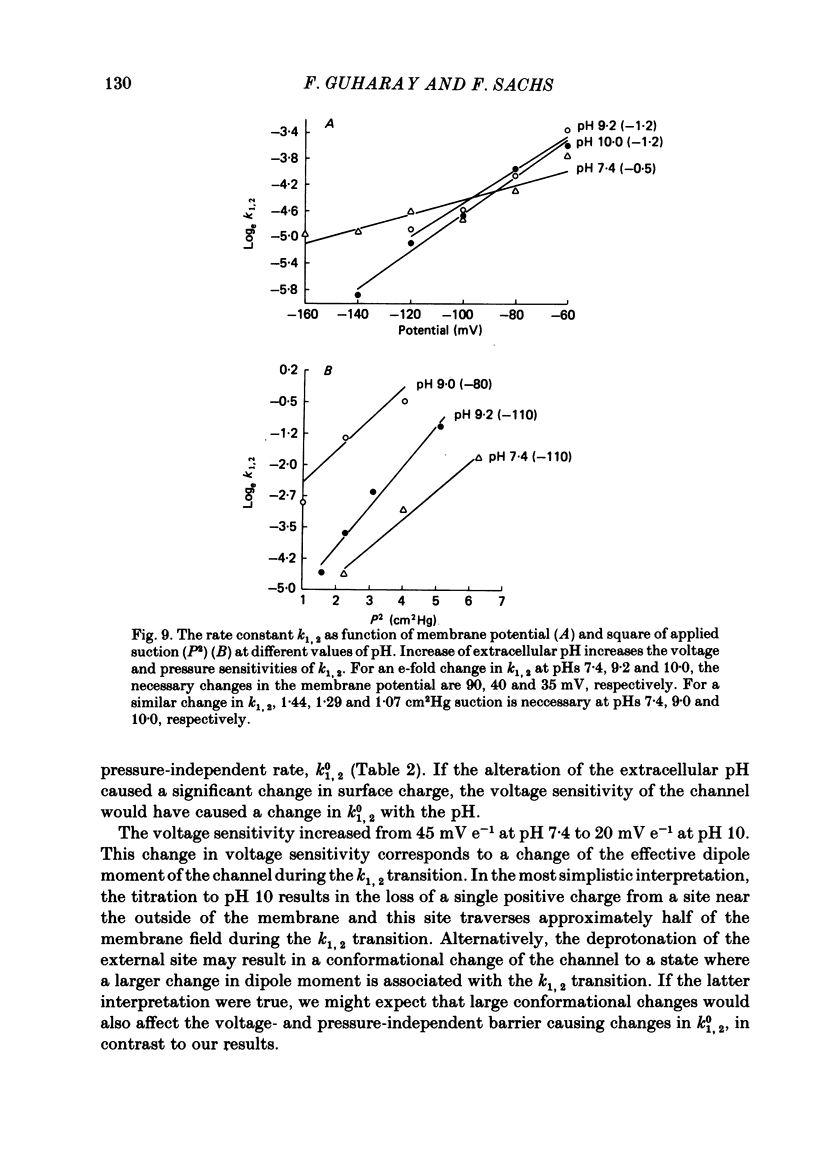
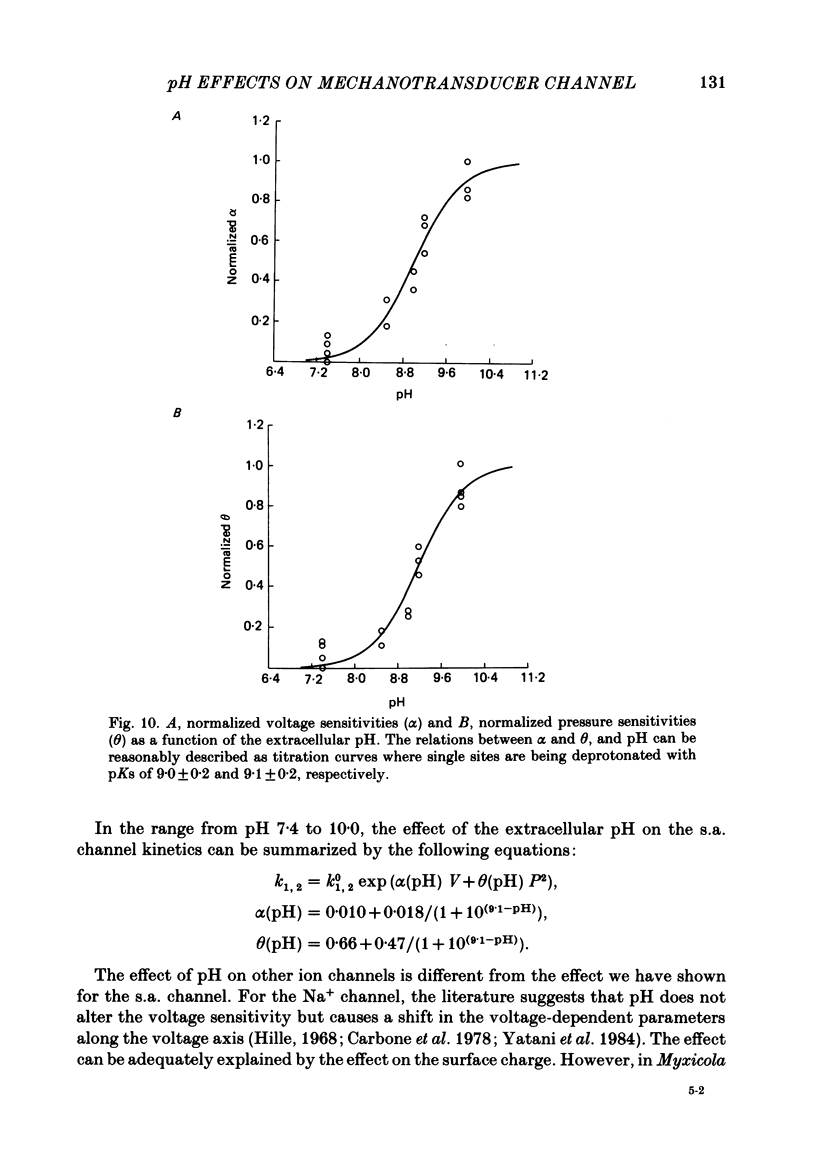
The membrane of tissue-cultured chick pectoral muscle contains an ionic channel which is activated by membrane tension. With 150 mM-external K+ and 150 mM-internal Na+, the channel has a conductance of 70 pS and a reversal potential of +30 mV. With 150 mM-external Na+ and 150 mM-internal K+ (normal gradient) the channel has a conductance of 35 pS and a reversal potential of -30 mV. The ratio of K+ permeability to Na+ permeability, PK:PNa, is 4 based upon reversal potentials and is 2 based upon conductance. Kinetic analysis of single-channel records indicates that there are one open (O) and three closed (C) states. When analysed according to a linear sequential model: C1-C2-C3-O4, only the rate constant that governs the C1-C2 transition (k1,2) is found to be affected by stretch or voltage. The effects of stretch and voltage on k1,2 can be summarized as k1,2 = k1,2(0) exp (alpha V + theta P2), where K1,2(0) is the voltage and stretch-independent part of the rate constant, alpha is the voltage sensitivity, V is the transmembrane potential, theta is the stretch sensitivity and P is the applied suction. Increasing extracellular pH from 7.4 to 10.0 increases both alpha and theta in a manner suggesting titration of site(s) with a pK of 9.1. A single lysine of N-terminal amino acid may be be responsible for modulating both the voltage and pressure responses. Extracellular pH does not affect k1,2(0), the voltage- and stretch-independent part of k1,2, suggesting that pH in the range 7.4-10 does not alter the local surface charge. The conductance and reversal potential of the s.a. channel are unaffected by pH, suggesting that the titrated site(s) is not close to the mouth of the channel.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A., Sachs F. Single-channel currents from acetylcholine receptors in embryonic chick muscle. Kinetic and conductance properties of gaps within bursts. Biophys J. 1984 Jan;45(1):187–198. doi: 10.1016/S0006-3495(84)84147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Kullberg R., Moody-Corbett F. Properties of non-junctional acetylcholine receptor channels on innervated muscle of Xenopus laevis. J Physiol. 1984 May;350:631–648. doi: 10.1113/jphysiol.1984.sp015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Ottoson D., Rydqvist B. Crayfish stretch receptor: an investigation with voltage-clamp and ion-sensitive electrodes. J Physiol. 1978 Nov;284:155–179. doi: 10.1113/jphysiol.1978.sp012533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Fioravanti R., Prestipino G., Wanke E. Action of extracellular pH on Na+ and K+ membrane currents in the giant axon of Loligo vulgaris. J Membr Biol. 1978 Nov 8;43(4):295–315. doi: 10.1007/BF01871693. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G., Lass Y. Evidence for acetylcholine receptor blockade by intracellular hydrogen ions in cultured chick myoballs. J Physiol. 1983 Oct;343:429–437. doi: 10.1113/jphysiol.1983.sp014901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Molgó J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978 Mar;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Alvarez O., Vergara C., Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83(3):273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- Mueller P., Pugh E. N., Jr Protons block the dark current of isolated retinal rods. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1892–1896. doi: 10.1073/pnas.80.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Bradley R. J., Dreyer F. The acetylcholine receptor at the neuromuscular junction. Physiol Rev. 1982 Oct;62(4 Pt 1):1271–1340. doi: 10.1152/physrev.1982.62.4.1271. [DOI] [PubMed] [Google Scholar]
- Sachs F., Auerbach A. Single-channel electrophysiology: use of the patch clamp. Methods Enzymol. 1983;103:147–176. doi: 10.1016/s0076-6879(83)03011-6. [DOI] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Davis F. A. Sensitivity of the sodium and potassium channels of Myxicola giant axons to changes in external pH. J Gen Physiol. 1976 Feb;67(2):185–195. doi: 10.1085/jgp.67.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuka M. The effects of pH on the conductance change evoked by iontophoresis in the frog neuromuscular junction. Pflugers Arch. 1977 Jul 19;369(3):239–244. doi: 10.1007/BF00582190. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Brown A. M., Akaike N. Effect of extracellular pH on sodium current in isolated, single rat ventricular cells. J Membr Biol. 1984;78(2):163–168. doi: 10.1007/BF01869203. [DOI] [PubMed] [Google Scholar]


