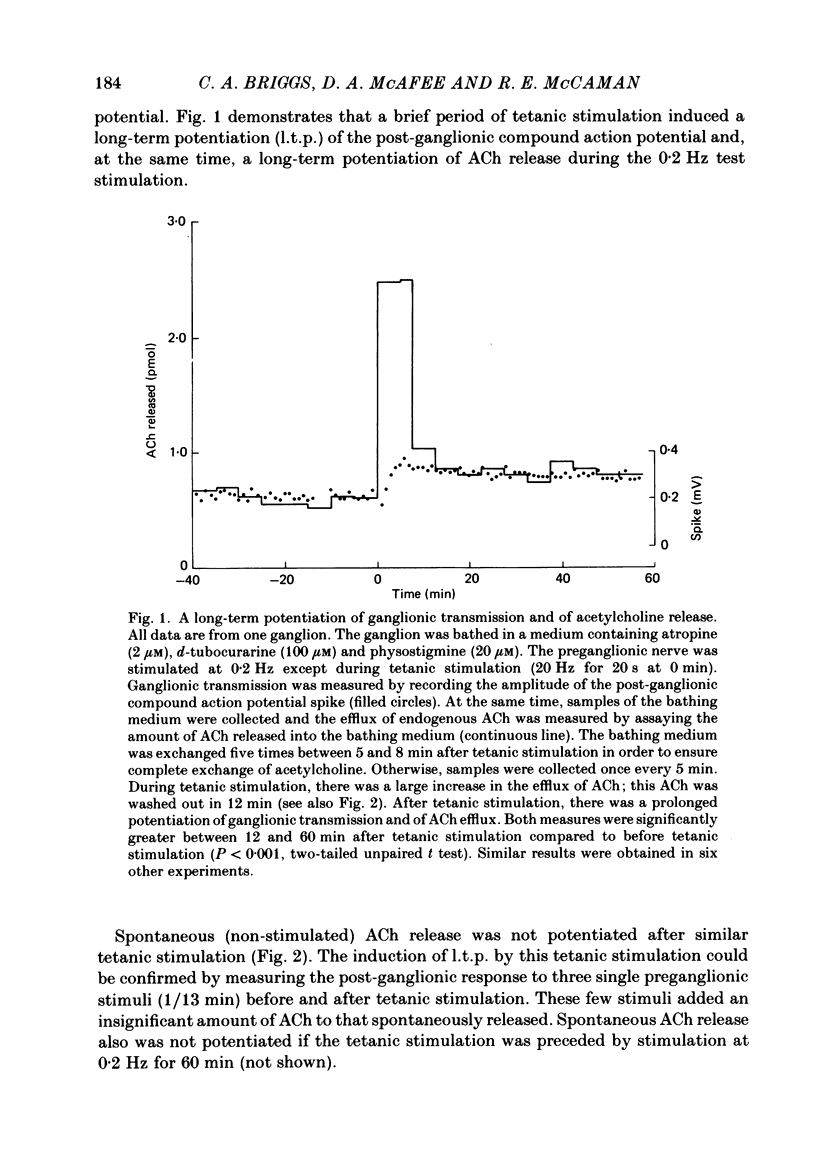
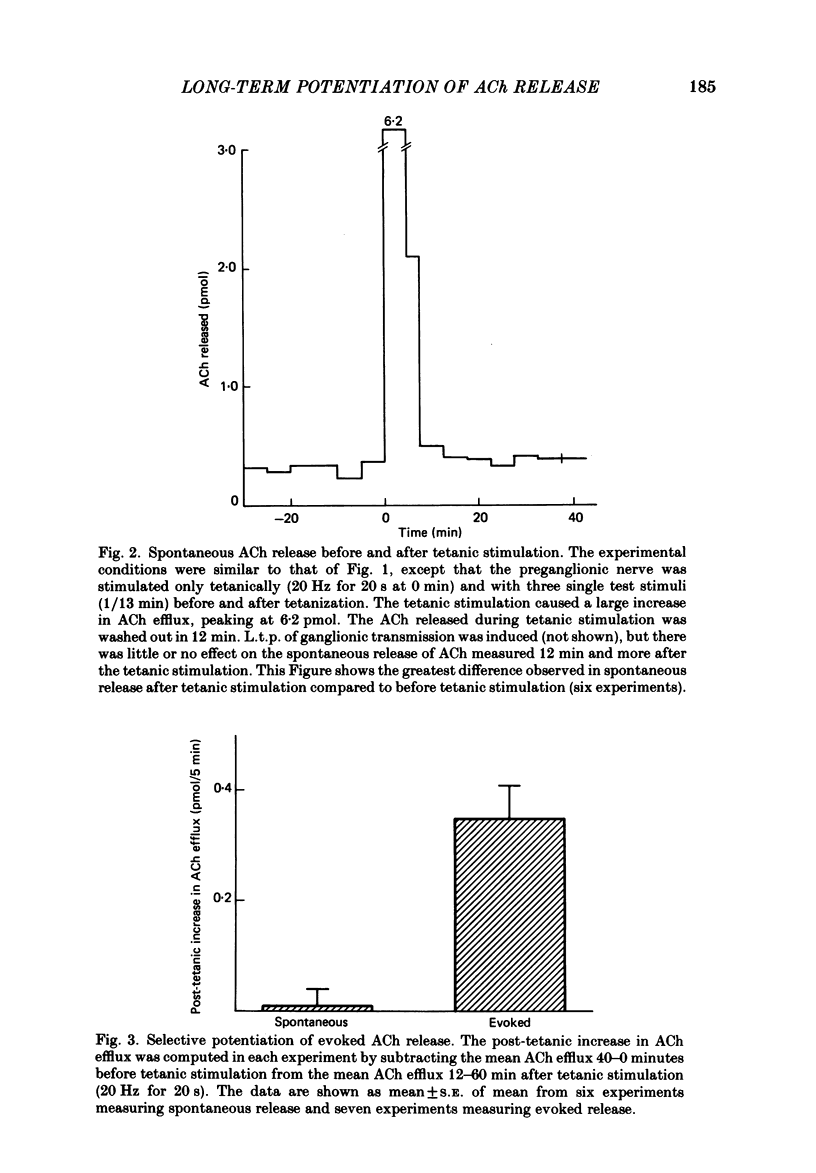
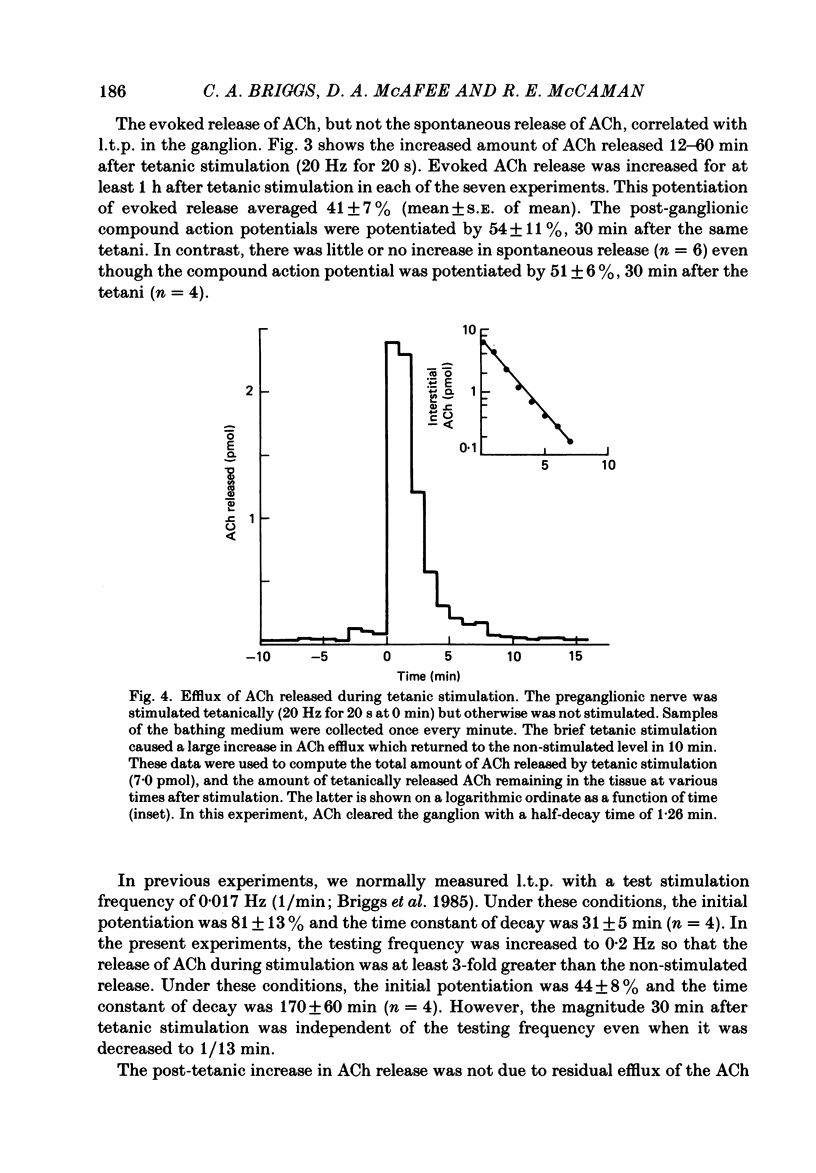
Abstract
The release of endogenous acetylcholine (ACh) from the in vitro rat superior cervical ganglion was measured by assaying the bathing medium. Simultaneously, synaptic transmission in the ganglion was assessed by recording post-ganglionic compound action potentials. A brief period of tetanic preganglionic stimulation (20 Hz for 20 s) induced a long-term potentiation of the post-ganglionic compound action potential. The same tetanic stimulation also consistently induced a long-term potentiation of stimulated ACh release. Spontaneous (non-stimulated) ACh release was not enhanced after tetanic stimulation. The content of ACh in the ganglion was not measurably increased after tetanic stimulation. These results suggest that the long-term increase in synaptic efficacy is due, at least in part, to an increase in the amount of ACh released by the afferent impulse.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birks R. I. A long-lasting potentiation of transmitter release related to an increase in transmitter stores in a sympathetic ganglion. J Physiol. 1977 Oct;271(3):847–862. doi: 10.1113/jphysiol.1977.sp012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. Regulation by patterned preganglionic neural activity of transmitter stores in a sympathetic ganglion. J Physiol. 1978 Jul;280:559–572. doi: 10.1113/jphysiol.1978.sp012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., McAfee D. A. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982 Mar 12;215(4538):1411–1413. doi: 10.1126/science.6278593. [DOI] [PubMed] [Google Scholar]
- Collier B., Kwok Y. N., Welner S. A. Increased acetylcholine synthesis and release following presynaptic activity in a sympathetic ganglion. J Neurochem. 1983 Jan;40(1):91–98. doi: 10.1111/j.1471-4159.1983.tb12657.x. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. M., McCaman R. E. The determination of picomole amounts of acetylcholine in mammalian brain. J Neurochem. 1973 Jan;20(1):1–8. doi: 10.1111/j.1471-4159.1973.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Hippocampal long-term potentiation: mechanisms and implications for memory. Based on an NRP Work Session. Neurosci Res Program Bull. 1982 Jun;20(5):613–769. [PubMed] [Google Scholar]
- Kumamoto E., Kuba K. Sustained rise in ACh sensitivity of a sympathetic ganglion cell induced by postsynaptic electrical activities. Nature. 1983 Sep 8;305(5930):145–146. doi: 10.1038/305145a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- McCaman R. E., Stetzler J. Radiochemical assay for ACh: modifications for sub-picomole measurements. J Neurochem. 1977 Mar;28(3):669–671. doi: 10.1111/j.1471-4159.1977.tb10442.x. [DOI] [PubMed] [Google Scholar]
- Sacchi O., Consolo S., Peri G., Prigioni I., Ladinsky H., Perri V. Storage and release of acetylcholine in the isolated superior cervical ganglion of the rat. Brain Res. 1978 Aug 11;151(3):443–456. doi: 10.1016/0006-8993(78)91078-8. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Malthe-Sørenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res. 1981 Mar 16;208(2):436–441. doi: 10.1016/0006-8993(81)90573-4. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]


