Abstract
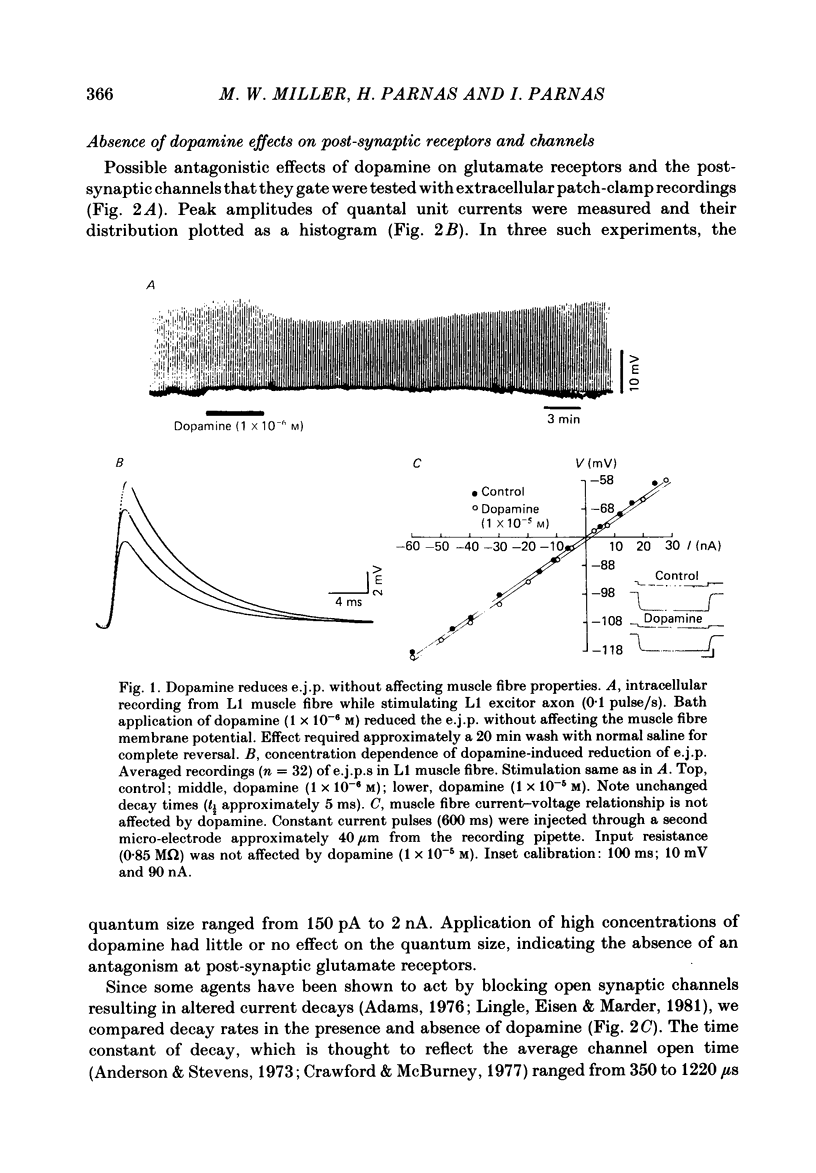
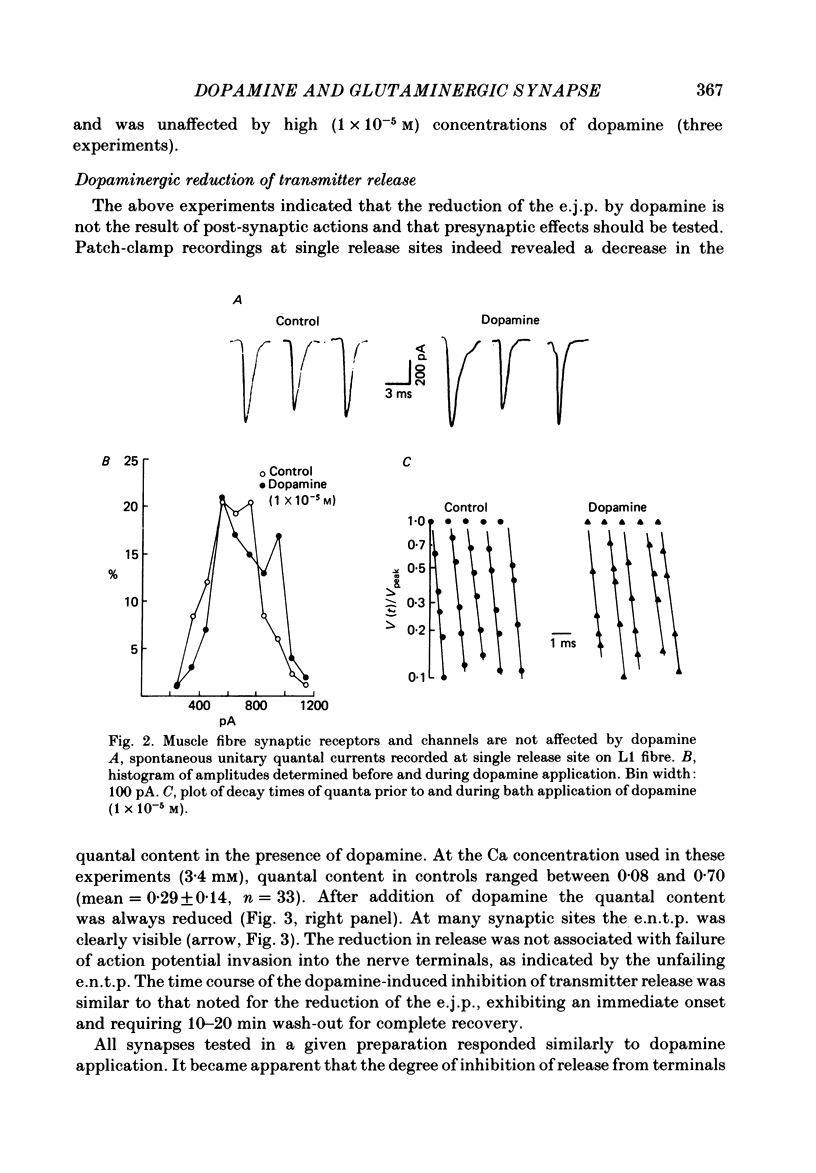
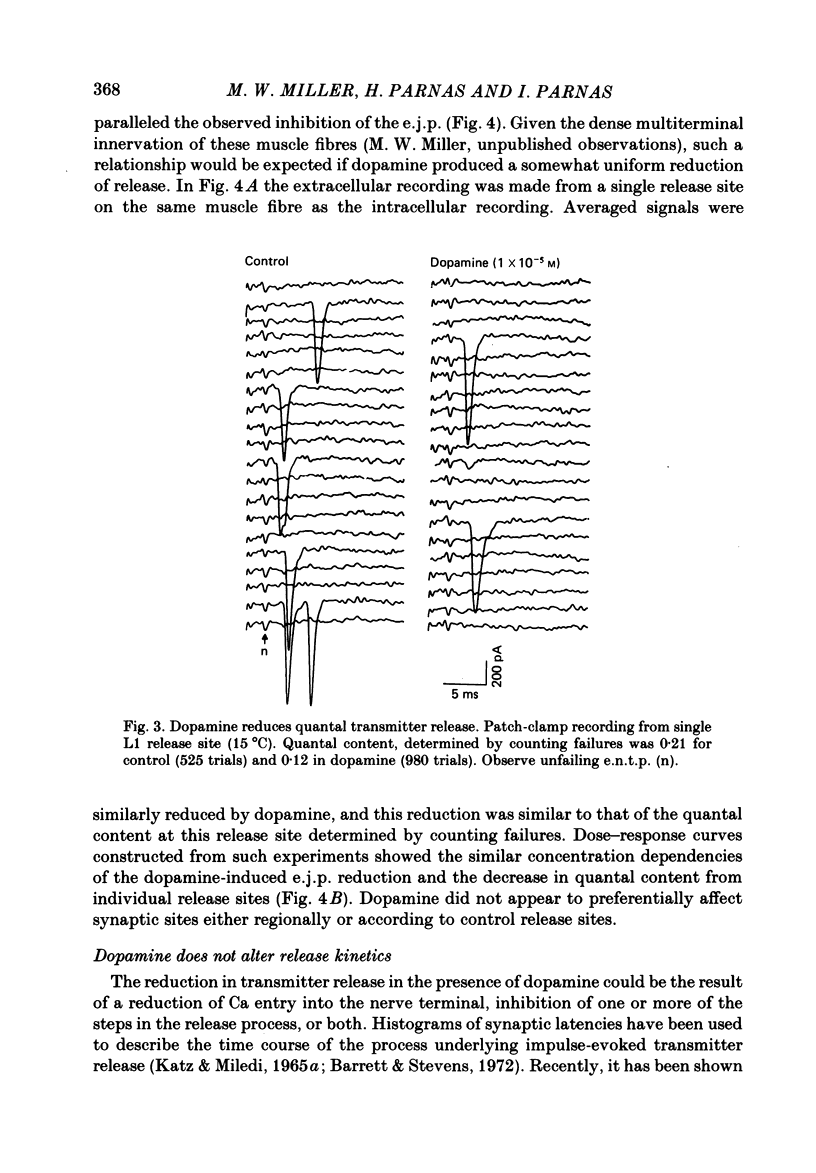
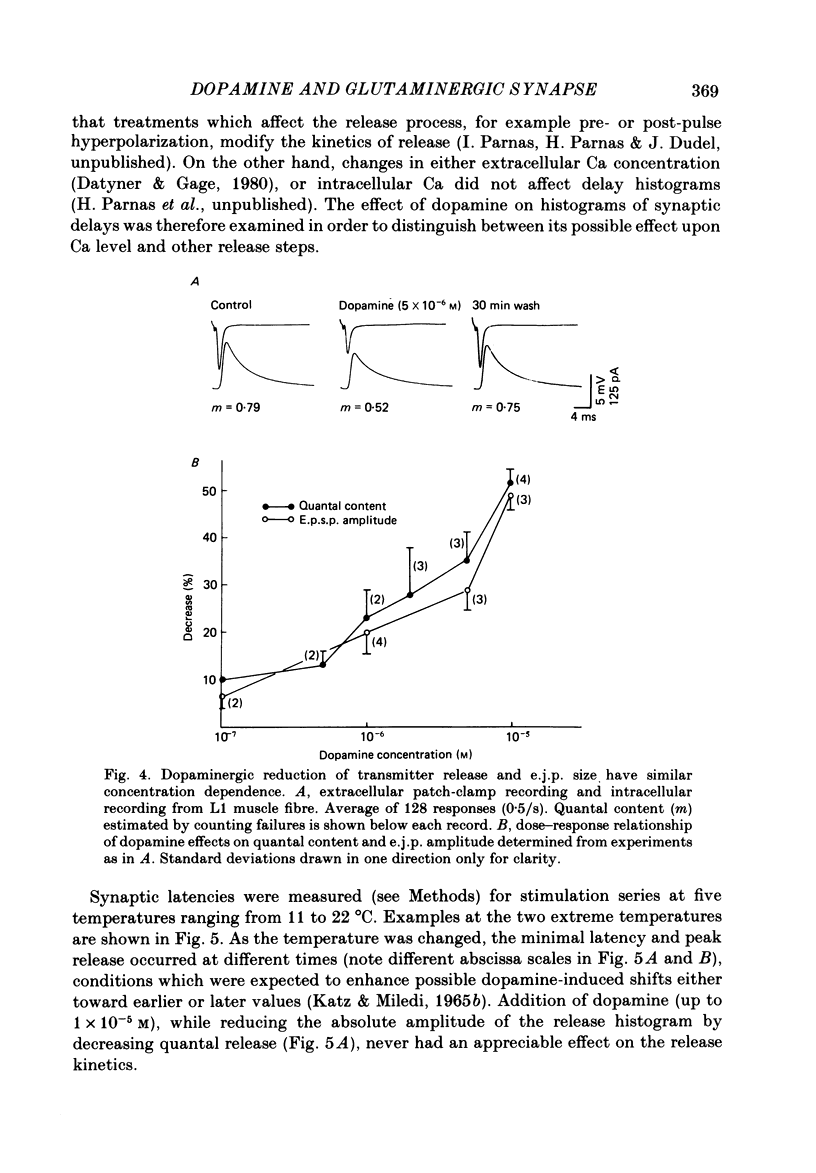
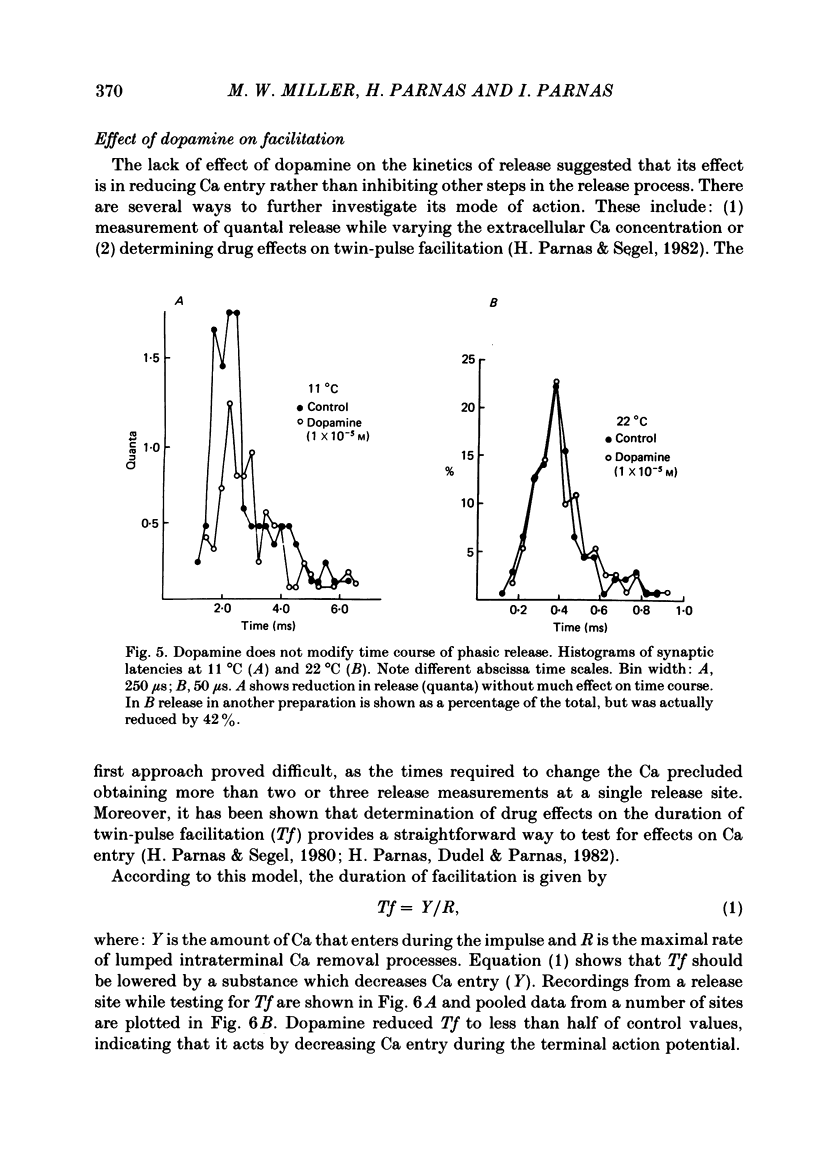
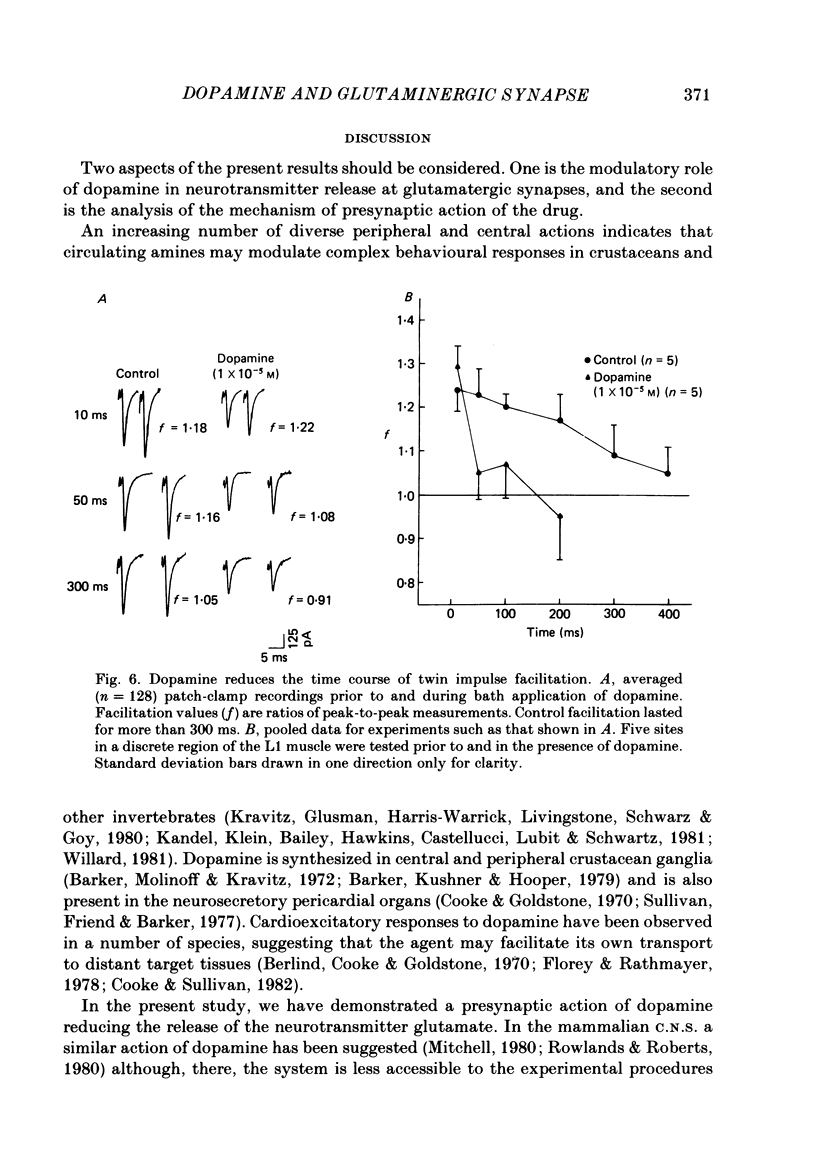
The action of the putative crustacean neurohormone dopamine was examined in the fast extensor musculature of the prawn with intracellular and extracellular recording techniques. Dopamine produced a concentration-dependent (10(-7)-10(-5) M) decrease in the size of the excitatory junctional potential (e.j.p.). It had no effect on the muscle fibre resting membrane potential or input resistance. High concentrations (10(-5)M) of dopamine had no effect on the amplitude distribution or decay time of quantal unit currents, indicating that the agent does not act by blocking post-synaptic receptors or channels. Bath application of dopamine reduced the quantal content at single release sites with a similar time course and concentration dependence as that observed for the e.j.p. Dopamine had no effect on histograms of synaptic delays determined over a 10 degree C range, indicating that it does not modify the time course of phasic neurosecretion. Twin-impulse facilitation experiments showed a marked decrease in the duration of facilitation in the presence of dopamine. These results are interpreted according to recent theoretical and experimental findings as indicating that the dopamine-induced reduction in transmitter release is produced by a decrease in the entry of Ca during the nerve terminal action potential.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. W., Barker D. L. Synaptic mechanisms that generate network oscillations in the absence of discrete postsynaptic potentials. J Exp Zool. 1981 Apr;216(1):187–191. doi: 10.1002/jez.1402160121. [DOI] [PubMed] [Google Scholar]
- Barker D. L., Kushner P. D., Hooper N. K. Synthesis of dopamine and octopamine in the crustacean stomatogastric nervous system. Brain Res. 1979 Jan 26;161(1):99–113. doi: 10.1016/0006-8993(79)90198-7. [DOI] [PubMed] [Google Scholar]
- Barker D. L., Molinoff P. B., Kravitz E. A. Octopamine in the lobster nervous system. Nat New Biol. 1972 Mar 15;236(63):61–63. doi: 10.1038/newbio236061a0. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlind A., Cooke I. M., Goldstone M. W. Do the monoamines in crab pericardial organs play a role in peptide neurosecretion? J Exp Biol. 1970 Dec;53(3):669–677. doi: 10.1242/jeb.53.3.669. [DOI] [PubMed] [Google Scholar]
- Breen C. A., Atwood H. L. Octopamine--a neurohormone with presynaptic activity-dependent effects at crayfish neuromuscular junctions. Nature. 1983 Jun 23;303(5919):716–718. doi: 10.1038/303716a0. [DOI] [PubMed] [Google Scholar]
- Cooke I. M., Goldstone M. W. Fluorescence localization of monoamines in crab neurosecretory structures. J Exp Biol. 1970 Dec;53(3):651–668. doi: 10.1242/jeb.53.3.651. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The termination of transmitter action at the crustacean excitatory neuromuscular junction. J Physiol. 1977 Jul;268(3):711–729. doi: 10.1113/jphysiol.1977.sp011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Parnas I., Parnas H. Neurotransmitter release and its facilitation in crayfish muscle. VI. Release determined by both, intracellular calcium concentration and depolarization of the nerve terminal. Pflugers Arch. 1983 Sep;399(1):1–10. doi: 10.1007/BF00652515. [DOI] [PubMed] [Google Scholar]
- Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflugers Arch. 1981 Jul;391(1):35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol. 1978 Apr;73:235–260. doi: 10.1242/jeb.73.1.235. [DOI] [PubMed] [Google Scholar]
- Fischer L., Florey E. Modulation of synaptic transmission and excitation-contraction coupling in the opener muscle of the crayfish, Astacus leptodactylus, by 5-hydroxytryptamine and octopamine. J Exp Biol. 1983 Jan;102:187–198. doi: 10.1242/jeb.102.1.187. [DOI] [PubMed] [Google Scholar]
- Florey E., Rathmayer M. The effects of octopamine and other amines on the heart and on neuromuscular transmission in decapod crustaceans: further evidence for a role as neurohormone. Comp Biochem Physiol C. 1978;61C(1):229–237. doi: 10.1016/0306-4492(78)90136-3. [DOI] [PubMed] [Google Scholar]
- Ganguly D. K., Das M. Effects of oxotremorine demonstrate presynaptic muscarinic and dopaminergic receptors on motor nerve terminals. Nature. 1979 Apr 12;278(5705):645–646. doi: 10.1038/278645a0. [DOI] [PubMed] [Google Scholar]
- Glusman S., Kravitz E. A. The action of serotonin on excitatory nerve terminals in lobster nerve-muscle preparations. J Physiol. 1982 Apr;325:223–241. doi: 10.1113/jphysiol.1982.sp014147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe R., Onodera K., Takeuchi A. On the quantal release of endogenous glutamate from the crayfish neuromuscular junction. J Physiol. 1982 Jan;322:529–539. doi: 10.1113/jphysiol.1982.sp014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe R., Onodera K., Takeuchi A. Release of glutamate from the crayfish neuromuscular junction. J Physiol. 1981 Mar;312:225–236. doi: 10.1113/jphysiol.1981.sp013625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz E. A., Glusman S., Harris-Warrick R. M., Livingstone M. S., Schwarz T., Goy M. F. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioural studies. J Exp Biol. 1980 Dec;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Modulatory actions of neurotransmitters. Annu Rev Neurosci. 1979;2:447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- Lingle C., Eisen J. S., Marder E. Block of glutamatergic excitatory synaptic channels by chlorisondamine. Mol Pharmacol. 1981 Mar;19(2):349–353. [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Calcium role in depolarization-secretion coupling: an aequorin study in squid giant synapse. Proc Natl Acad Sci U S A. 1975 Jan;72(1):187–190. doi: 10.1073/pnas.72.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. I. Saturation kinetics of release, and of entry and removal of calcium. Pflugers Arch. 1982 Mar;393(1):1–14. doi: 10.1007/BF00582384. [DOI] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. A theoretical explanation for some effects of calcium on the facilitation of neurotransmitter release. J Theor Biol. 1980 May 7;84(1):3–29. doi: 10.1016/s0022-5193(80)81035-6. [DOI] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. Ways to discern the presynaptic effect of drugs on neurotransmitter release. J Theor Biol. 1982 Feb 21;94(4):923–941. doi: 10.1016/0022-5193(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Parnas I., Atwood H. L. Phasic and tonic neuromuscular systems in the abdominal extensor muscles of the crayfish and rock lobster. Comp Biochem Physiol. 1966 Aug;18(4):701–723. doi: 10.1016/0010-406x(66)90206-4. [DOI] [PubMed] [Google Scholar]
- Parnas I., Dagan D. Electrical and mechanical properties of abdominal extensor muscles of the prawn Palaemon elegans. Comp Biochem Physiol. 1969 Jan;28(1):359–369. doi: 10.1016/0010-406x(69)91349-8. [DOI] [PubMed] [Google Scholar]
- Parnas I., Dudel J., Parnas H. Depolarization dependence of the kinetics of phasic transmitter release at the crayfish neuromuscular junction. Neurosci Lett. 1984 Sep 7;50(1-3):157–162. doi: 10.1016/0304-3940(84)90479-8. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H., Dudel J. Neurotransmitter release and its facilitation in crayfish. II. Duration of facilitation and removal processes of calcium from the terminal. Pflugers Arch. 1982 May;393(3):232–236. doi: 10.1007/BF00584075. [DOI] [PubMed] [Google Scholar]
- Rowlands G. F., Roberts P. J. Activation of dopamine receptors inhibits calcium-dependent glutamate release from cortico--striatal terminals in vitro. Eur J Pharmacol. 1980 Mar 21;62(2-3):241–242. doi: 10.1016/0014-2999(80)90285-x. [DOI] [PubMed] [Google Scholar]
- Sullivan R. E., Friend B. J., Barker D. L. Structure and function of spiny lobster ligamental nerve plexuses: evidence for synthesis, storage, and secretion of biogenic amines. J Neurobiol. 1977 Nov;8(6):581–605. doi: 10.1002/neu.480080607. [DOI] [PubMed] [Google Scholar]
- Trimble D. L., Barker D. L. Activation by dopamine of patterned motor output from the buccal ganglia of Helisoma trivolvis. J Neurobiol. 1984 Jan;15(1):37–48. doi: 10.1002/neu.480150105. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Cohen J. L., Kupfermann I. Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol. 1978 Jan;41(1):181–203. doi: 10.1152/jn.1978.41.1.181. [DOI] [PubMed] [Google Scholar]
- Wieland S. J., Gelperin A. Dopamine elicits feeding motor program in Limax maximus. J Neurosci. 1983 Sep;3(9):1735–1745. doi: 10.1523/JNEUROSCI.03-09-01735.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard A. L. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J Neurosci. 1981 Sep;1(9):936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


