Abstract
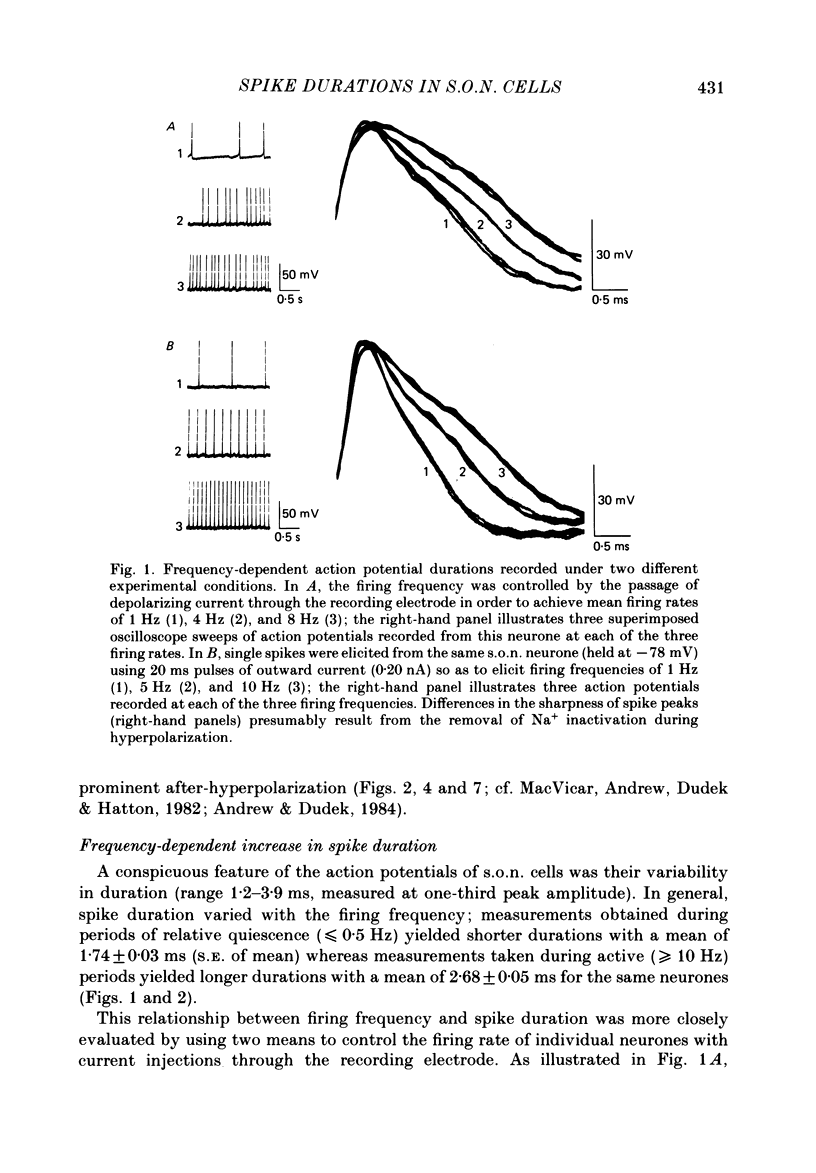
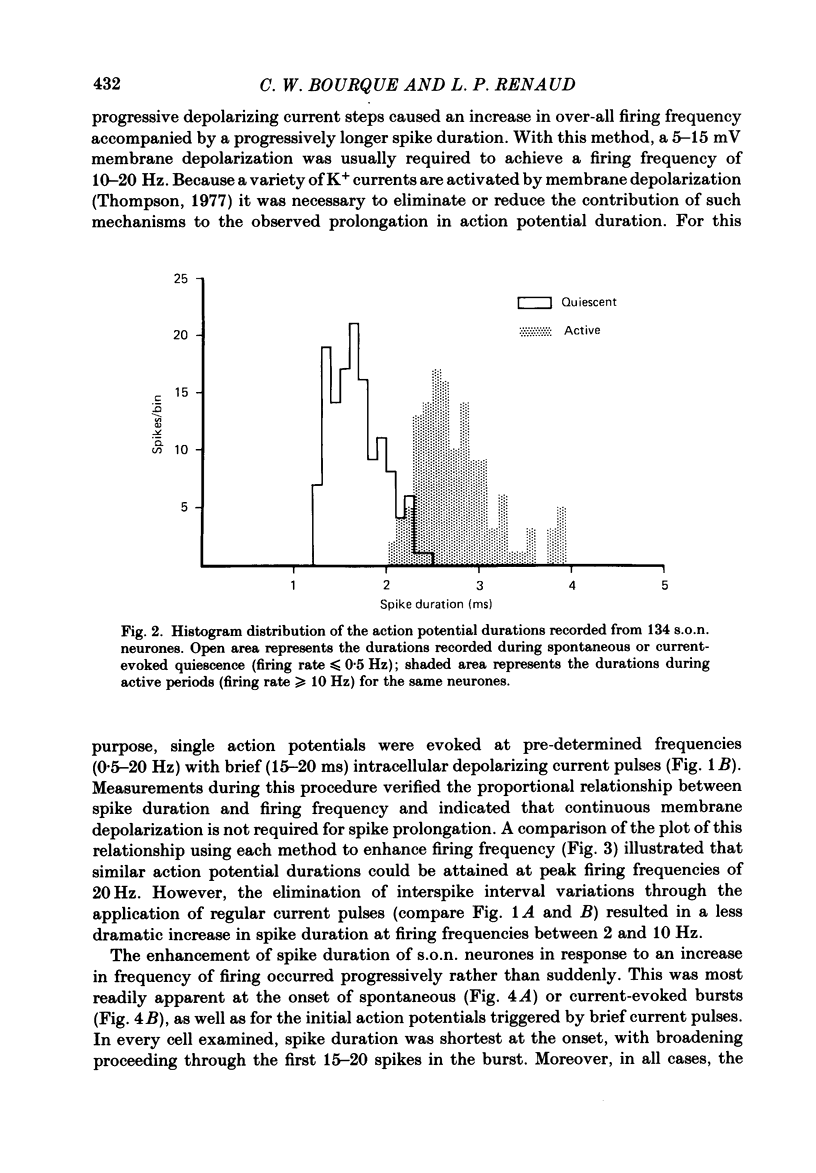
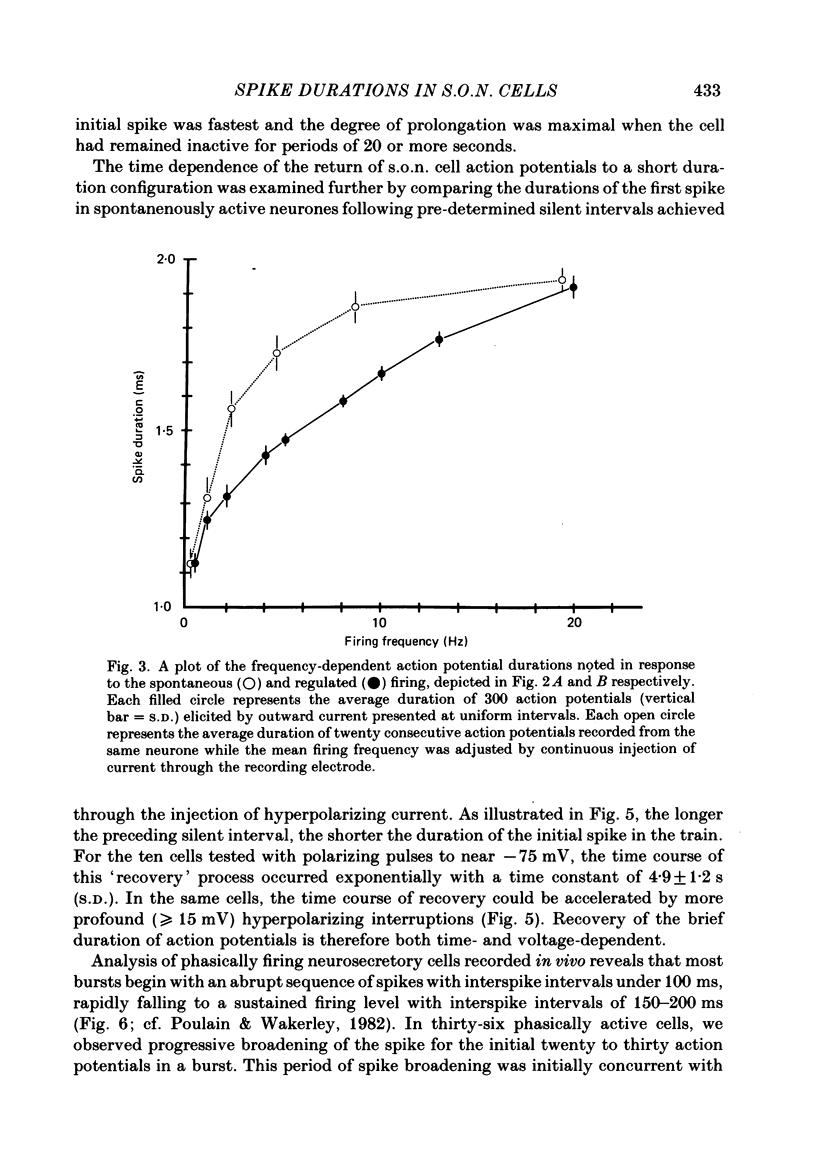
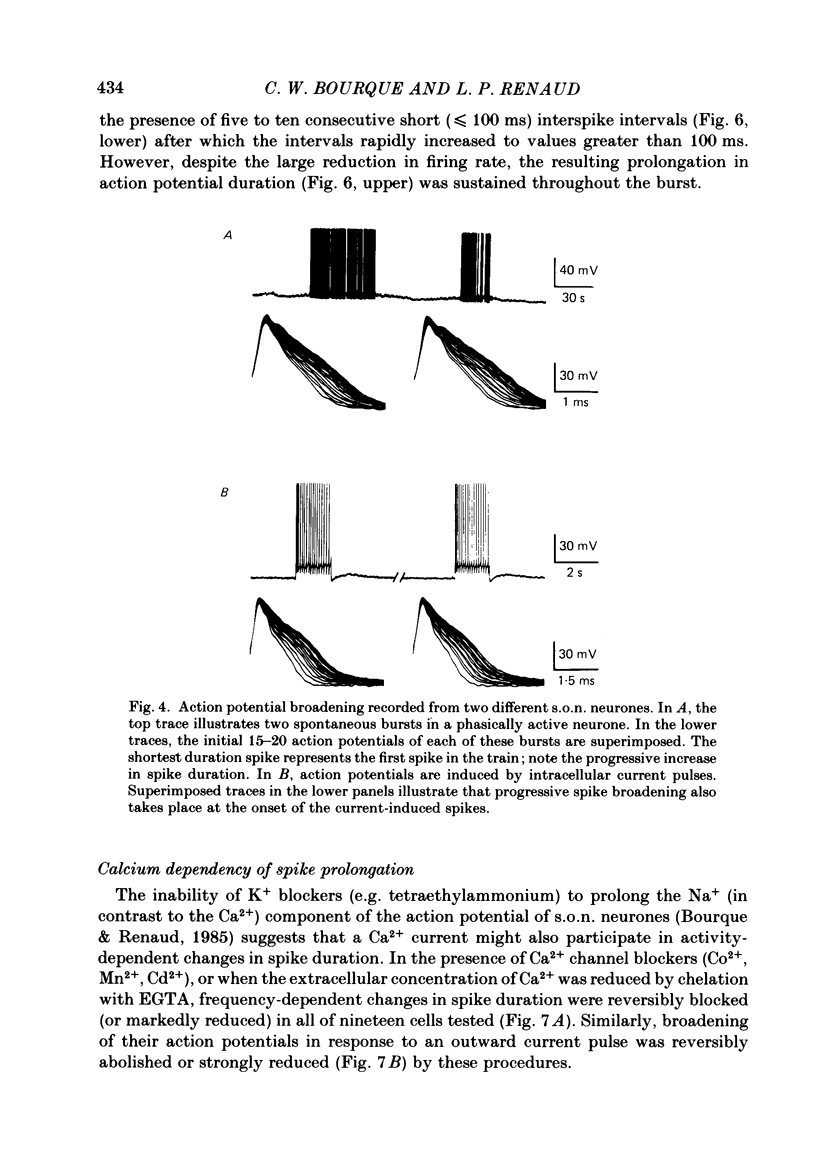
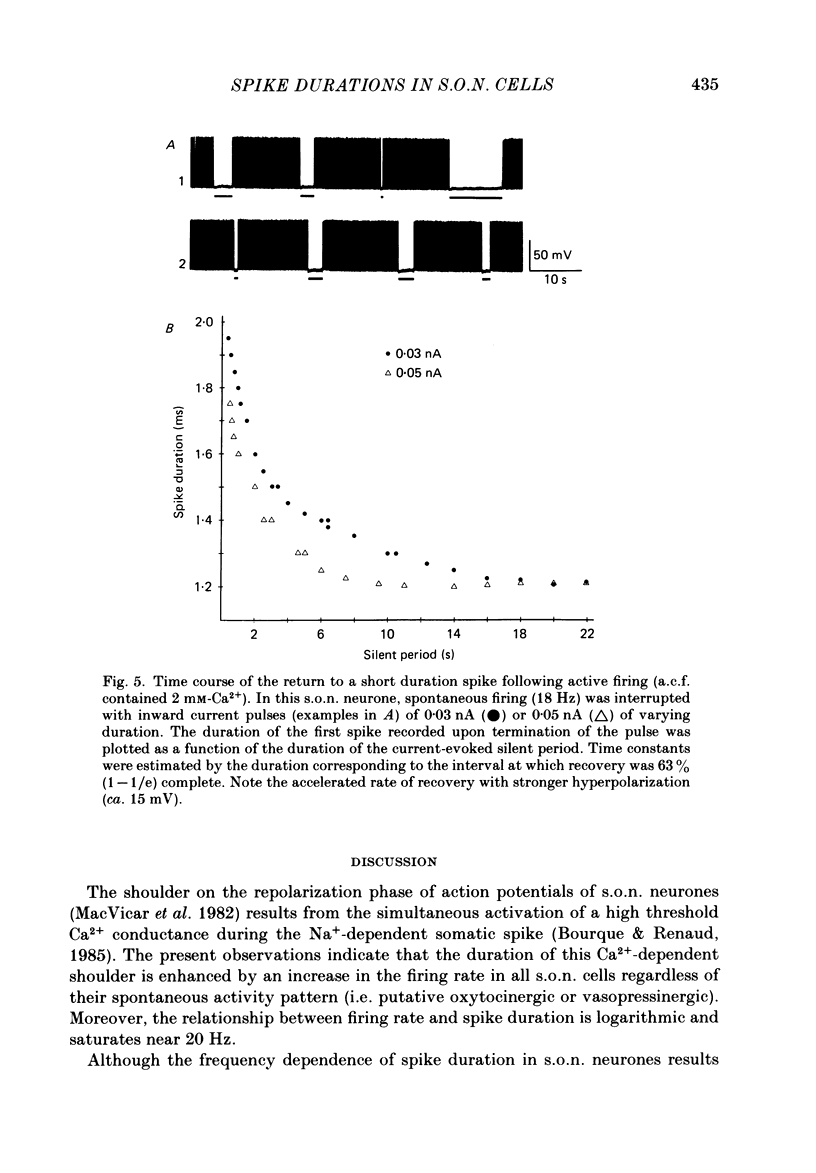
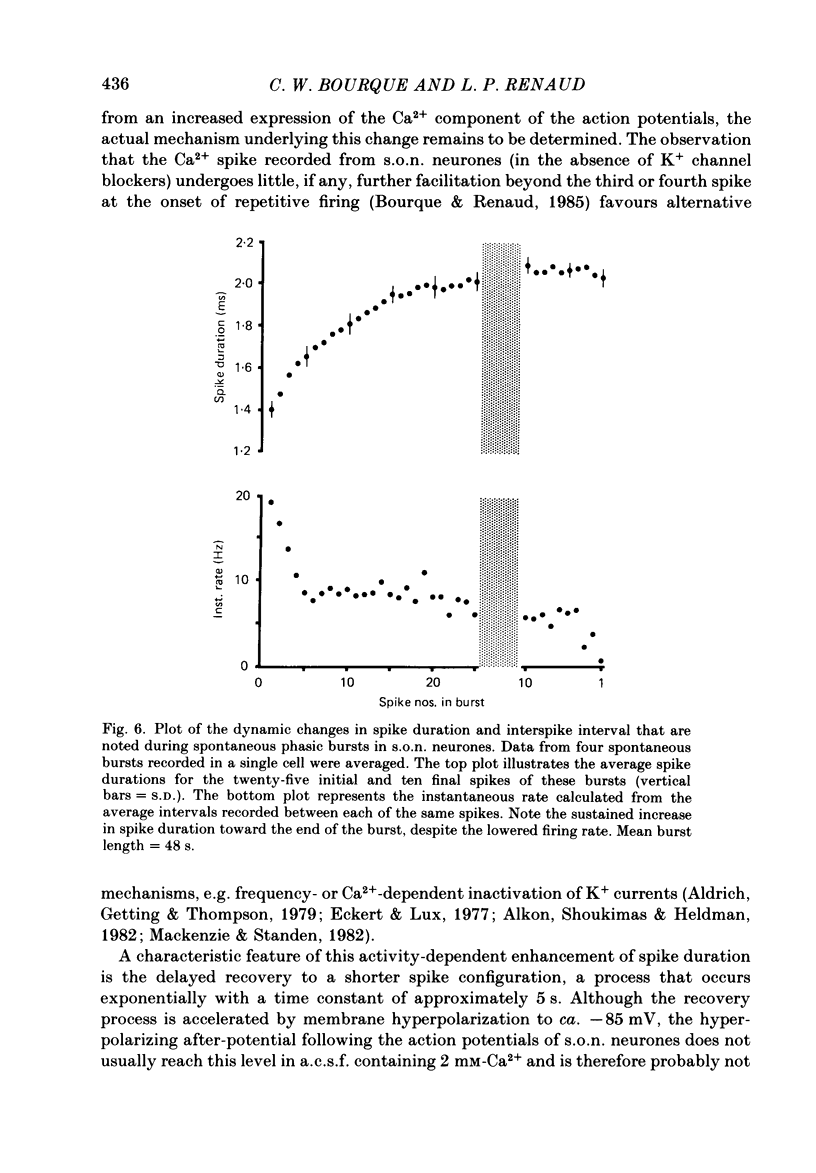
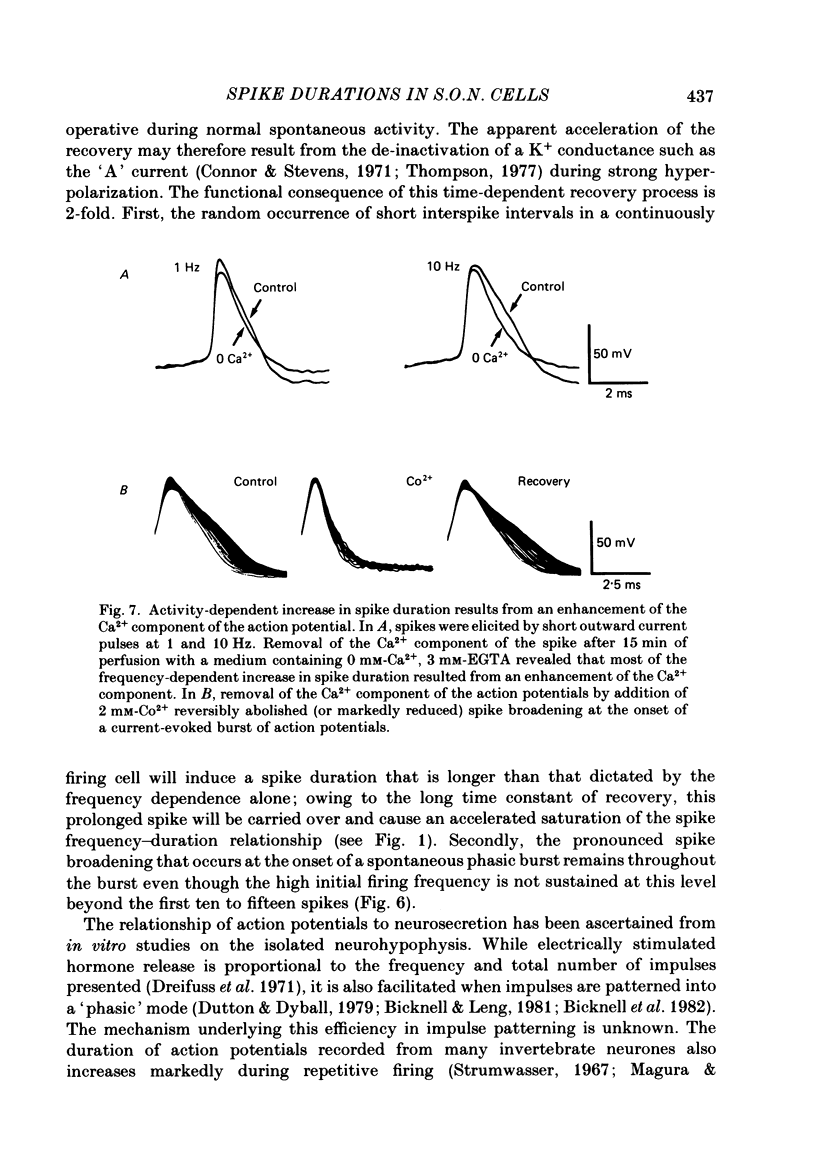
Action potential durations, measured at one-third peak amplitude, were examined during intracellular recordings in 134 supraoptic nucleus neurones maintained in vitro in perfused hypothalamic explants. Spike durations ranged between 1.2 and 3.9 ms and were dependent on firing frequency. Shortest measurements (1.74 +/- 0.03 ms; mean +/- S.E. of mean) were obtained during relative quiescence, i.e. less than or equal to 0.5 Hz. A gradual increase in firing frequency through continuous injection of depolarizing current prolonged spike duration, with maximum levels (2.68 +/- 0.05 ms) achieved at 20 Hz. When interspike interval variability was eliminated and firing was more precisely regulated by brief 15-20 ms intracellular current pulses given at pre-determined frequencies, a proportional relationship between increasing spike duration and firing frequency was retained but the change in spike duration at frequencies between 2 and 10 Hz was less pronounced. Once action potentials had achieved the long duration configuration, their return to the shorter duration took place gradually during any succeeding silent interval with a time constant of 4.9 s. Action potential broadening occurred progressively and was most pronounced at the onset of spontaneous or current-induced bursts. In thirty-six phasically active neurones, spike broadening at the onset of a burst was concurrent with the presence of 5-10 consecutive short (less than or equal to 100 ms) interspike intervals; thereafter, despite a greater than 50% reduction in firing frequency, action potential durations remained prolonged throughout the burst. In all of nineteen cells tested, frequency-dependent changes in spike duration were reversibly decreased or blocked by Cd2+, Co2+ and Mn2+, or when CaCl2 was exchanged for equimolar amounts of EGTA in the perfusion medium. These observations indicate that a Ca2+ conductance contributes to frequency- and firing-pattern-dependent changes in spike duration in rat supraoptic nucleus neurones.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol. 1979 Jun;291:531–544. doi: 10.1113/jphysiol.1979.sp012829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L., Shoukimas J. J., Heldman E. Calcium-mediated decrease of a voltage-dependent potassium current. Biophys J. 1982 Dec;40(3):245–250. doi: 10.1016/S0006-3495(82)84479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. J Physiol. 1984 Aug;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981 Nov;33(5):295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. Calcium-dependent action potentials in rat supraoptic neurosecretory neurones recorded in vitro. J Physiol. 1985 Jun;363:419–428. doi: 10.1113/jphysiol.1985.sp015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kalnins I., Kelly J. S., Ruf K. B. Action potentials and release of neurohypophysial hormones in vitro. J Physiol. 1971 Jul;215(3):805–817. doi: 10.1113/jphysiol.1971.sp009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. Calcium-dependent depression of a late outward current in snail neurons. Science. 1977 Jul 29;197(4302):472–475. doi: 10.1126/science.17921. [DOI] [PubMed] [Google Scholar]
- Gola M. Evolution de la frome des potentiels d'action par stimulations répétitives. Analyse par la méthode du voltage imposé (neurones d'Helix pomatia. Pflugers Arch. 1974 Feb 4;346(2):121–140. doi: 10.1007/BF00587012. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Andrew R. D., Dudek F. E., Hatton G. I. Synaptic inputs and action potentials of magnocellular neuropeptidergic cells: intracellular recording and staining in slices of rat hypothalamus. Brain Res Bull. 1982 Jan;8(1):87–93. doi: 10.1016/0361-9230(82)90031-4. [DOI] [PubMed] [Google Scholar]
- Mackenzie E., Standen N. B. The effects of stimulation rate on calcium-dependent action potentials recorded from chick embryo heart cell aggregates. J Physiol. 1982 Mar;324:1–10. doi: 10.1113/jphysiol.1982.sp014096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Senseman D. M., Gainer H. Optical recording of action potentials from vertebrate nerve terminals using potentiometric probes provides evidence for sodium and calcium components. Nature. 1983 Nov 3;306(5938):36–40. doi: 10.1038/306036a0. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]


