Abstract
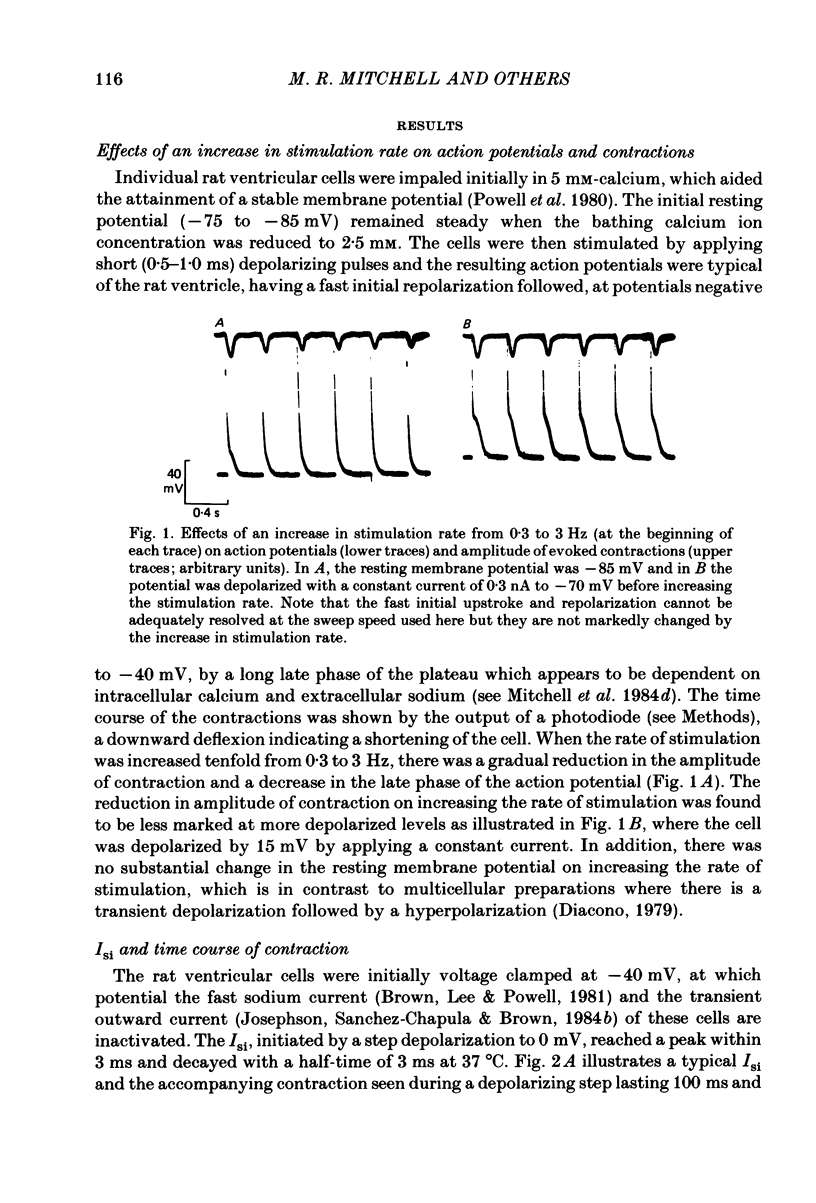
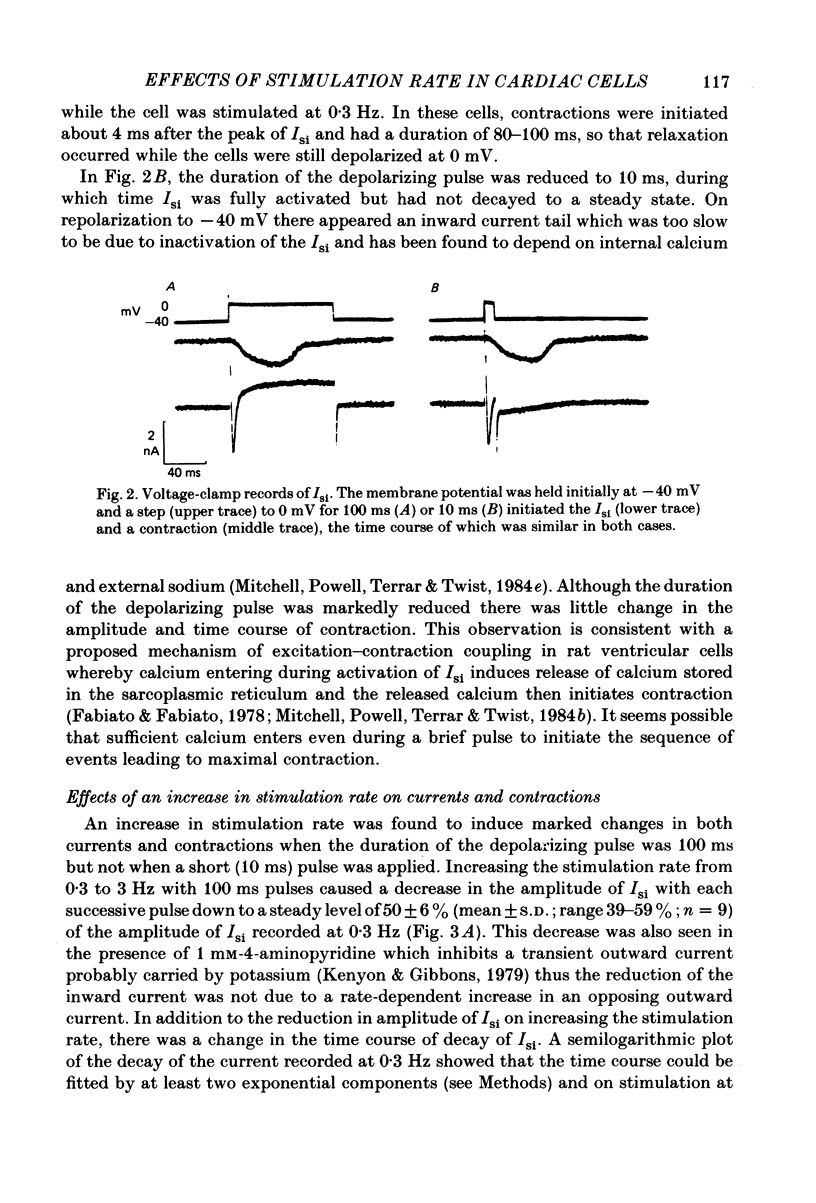
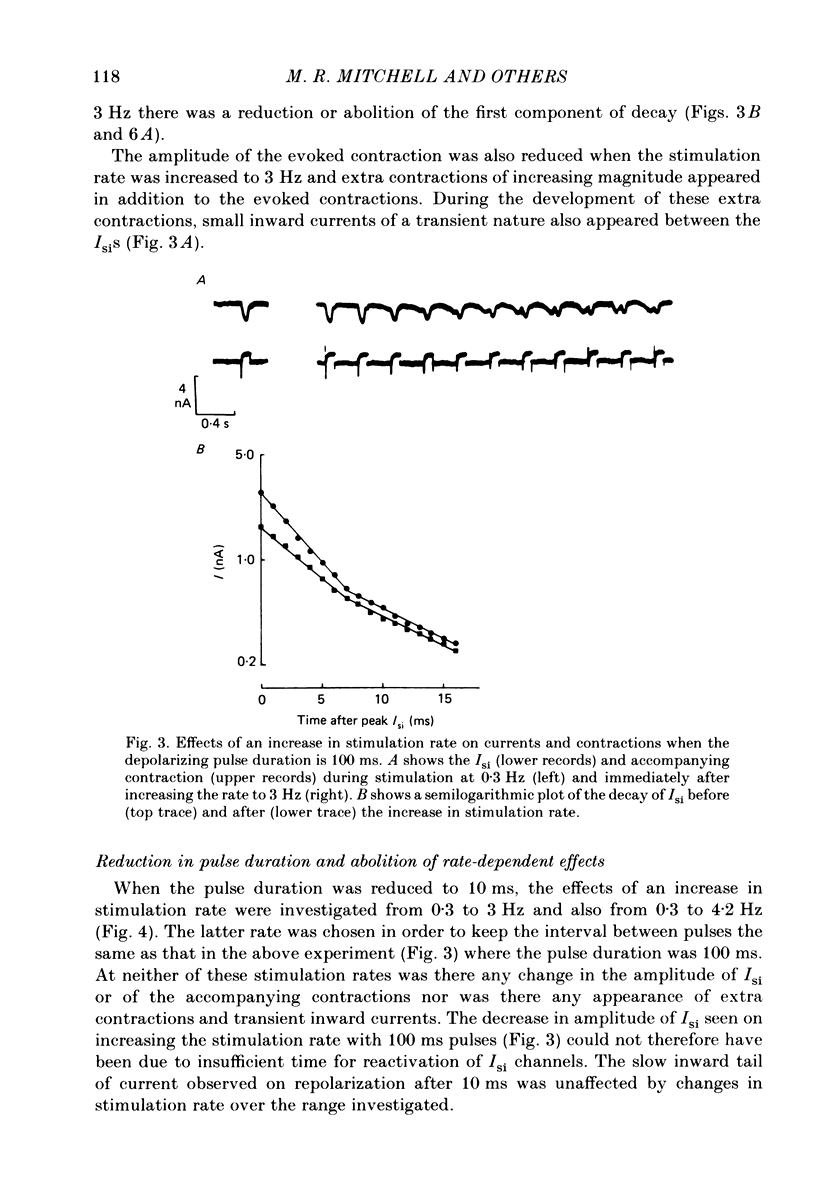
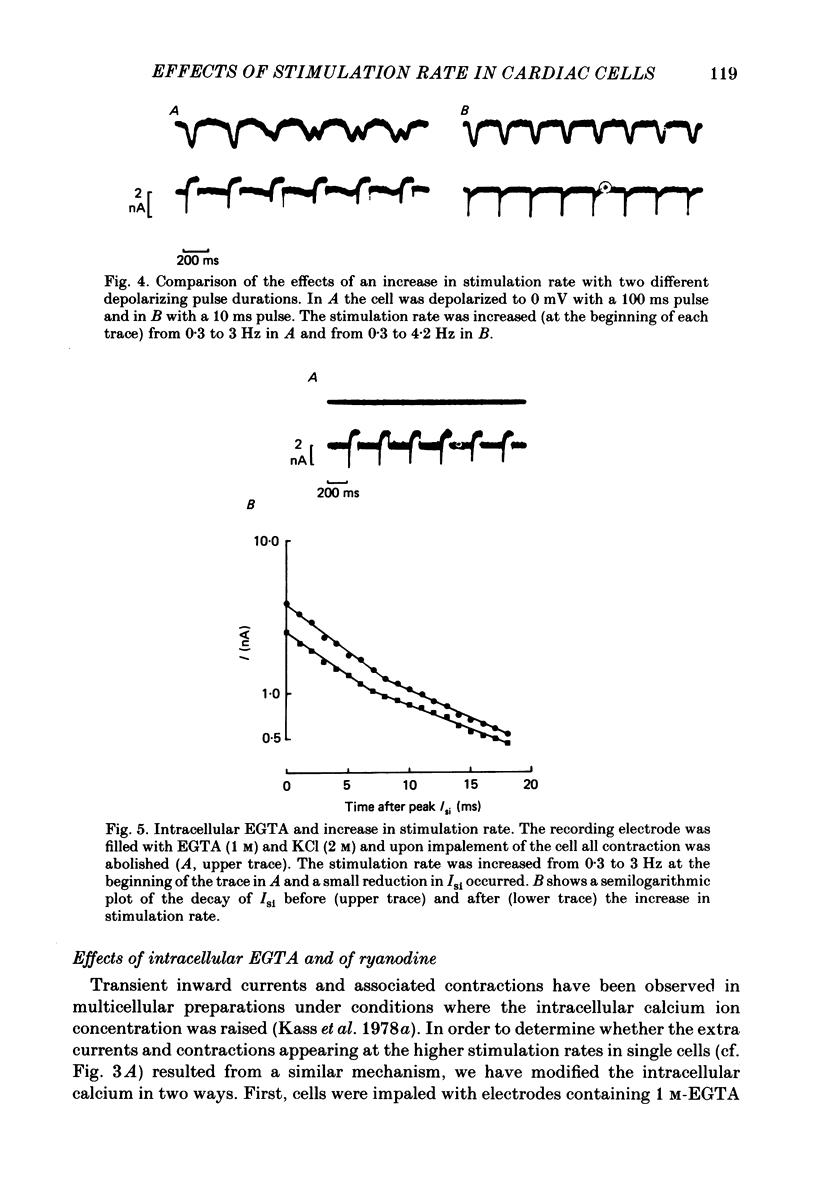
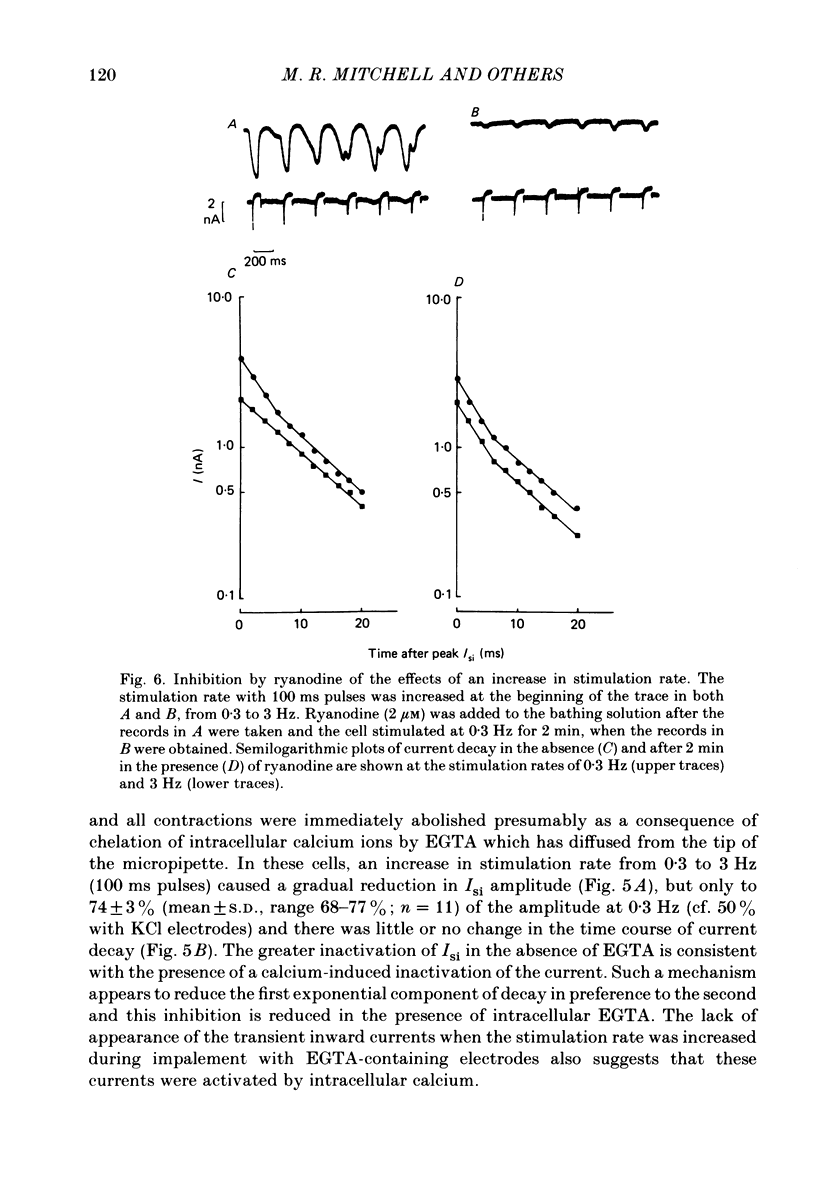
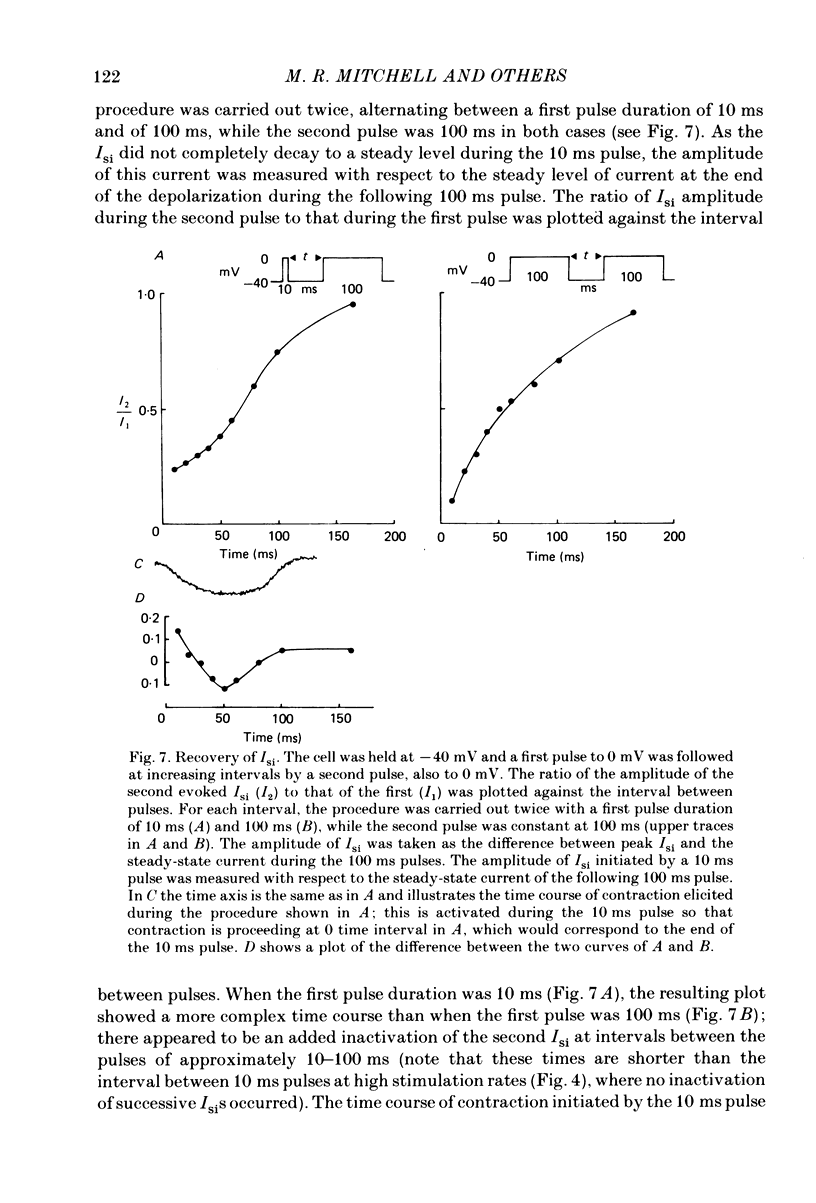
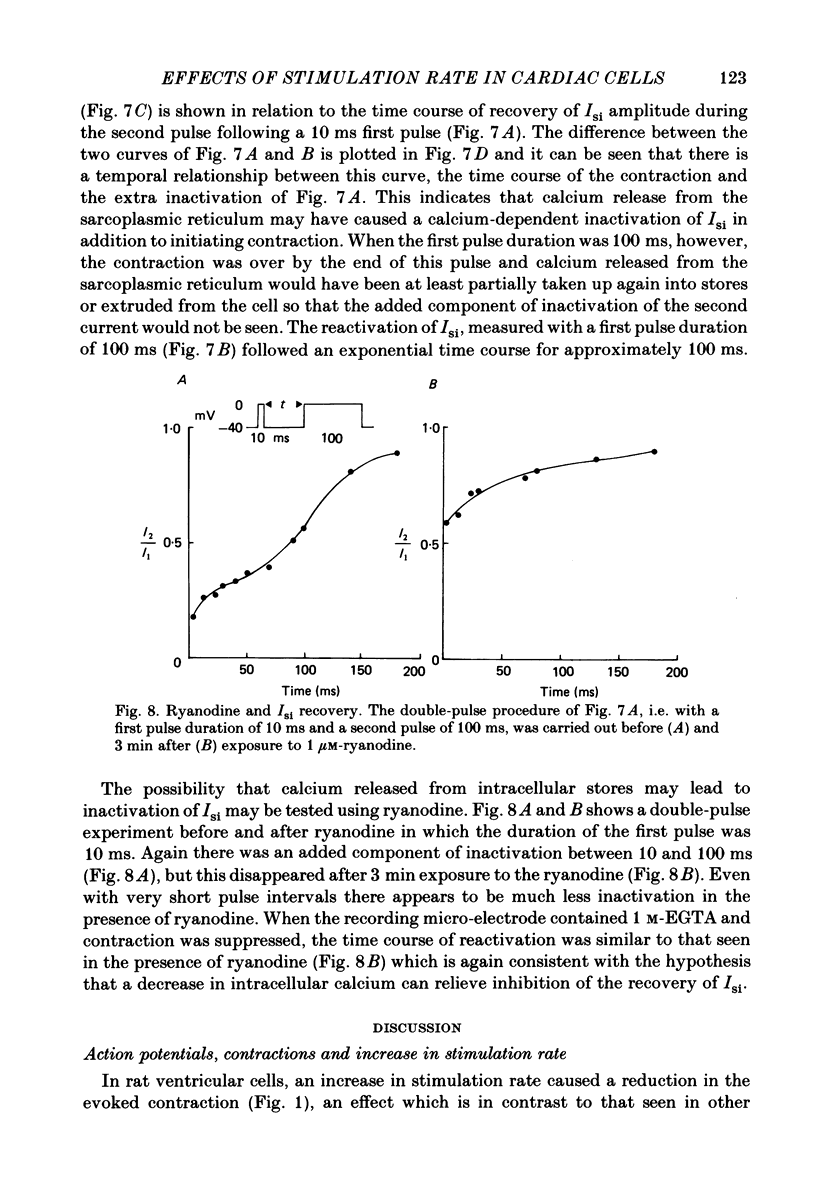
The effects of a change in stimulation rate on electrical activity and accompanying contraction were investigated in ventricular cells isolated from rat heart; the cells were stimulated to contract either by brief depolarization pulses which evoked action potentials, or, under voltage-clamp conditions, by step depolarizations. An increase in stimulation rate from 0.3 to 3 Hz resulted in a gradual reduction in the amplitude of contraction and attenuation of the late phase of the action potential. These changes were less marked at more depolarized potentials. The ventricular cells were voltage clamped at -40 mV and initially stimulated at 0.3 Hz by step depolarizations to 0 mV for 10 or 100 ms, which activated the second inward current (Isi) and an accompanying contraction. The amplitude and time course of contraction were similar with the two pulse durations. When the duration of the depolarization was 100 ms, an increase in stimulation rate to 3 Hz caused a gradual decline in the amplitude of Isi and of the evoked contraction; at the same time extra contractions and small, transient inward currents appeared in addition to the evoked contractions and Isis. There was a reduction in the early component of decay of Isi at 3 Hz. With a depolarizing pulse duration of 10 ms, an increase in stimulation rate to 3 or to 4.2 Hz did not change the amplitude of the evoked Isi or contraction and no extra contractions or currents appeared. Intracellular EGTA abolished all contractions in the cells and an increase in the rate of stimulation with 100 ms pulses did not then induce transient inward currents. There was some decrease in the Isi amplitude but this was not as marked as in the absence of EGTA and the time course of current decay was similar at the two rates. Ryanodine prevented the appearance of extra contractions and currents when the stimulation rate was increased to 3 Hz and, as in the presence of intracellular EGTA, there was a small decrease in Isi amplitude while the time course of decay was similar at the two stimulation rates. The time course of recovery of Isi from inactivation, as shown by a double-pulse procedure, was altered when the duration of the first pulse was reduced from 100 to 10 ms, an extra inactivation of Isi being seen at pulse intervals of 20-100 ms. This extra component of inactivation was not seen with intracellular EGTA or in the presence of ryanodine.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W. Characteristics of sodium and calcium conductance changes produced by membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:143–161. doi: 10.1113/jphysiol.1979.sp012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Orchard C. H. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984 Jul;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Jewell B. R., Wood E. H. Studies of the contractility of mammalian myocardium at low rates of stimulation. J Physiol. 1976 Jan;254(1):1–17. doi: 10.1113/jphysiol.1976.sp011217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium inactivation in skeletal muscle fibres of the stick insect, Carausius morosus. J Physiol. 1982 Sep;330:349–372. doi: 10.1113/jphysiol.1982.sp014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Kimura J., Noble D., Noble S. J., Taupignon A. The slow inward current, isi, in the rabbit sino-atrial node investigated by voltage clamp and computer simulation. Proc R Soc Lond B Biol Sci. 1984 Sep 22;222(1228):305–328. doi: 10.1098/rspb.1984.0066. [DOI] [PubMed] [Google Scholar]
- Diacono J. Suggestive evidence for the activation of an electrogenic sodium pump in stimulated rat atria: apparent discrepancy between the pump inhibition and the positive inotropic response induced by ouabain. J Mol Cell Cardiol. 1979 Jan;11(1):5–30. doi: 10.1016/0022-2828(79)90449-8. [DOI] [PubMed] [Google Scholar]
- Eckert R., Ewald D. Inactivation of calcium conductance characterized by tail current measurements in neurones of Aplysia californica. J Physiol. 1983 Dec;345:549–565. doi: 10.1113/jphysiol.1983.sp014996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Jóhannsson M. The contractile state of rabbit papillary muscle in relation to stimulation frequency. J Physiol. 1976 Jan;254(3):565–581. doi: 10.1113/jphysiol.1976.sp011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Forester G. V., Mainwood G. W. Interval dependent inotropic effects in the rat myocardium and the effect of calcium. Pflugers Arch. 1974;352(3):189–196. doi: 10.1007/BF00590484. [DOI] [PubMed] [Google Scholar]
- Hajdu S. Mechanism of the Woodworth staircase phenomenon in heart and skeletal muscle. Am J Physiol. 1969 Jan;216(1):206–214. doi: 10.1152/ajplegacy.1969.216.1.206. [DOI] [PubMed] [Google Scholar]
- Hess P., Wier W. G. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of caffeine on the intracellular [Ca2+] transient, membrane currents, and contraction. J Gen Physiol. 1984 Mar;83(3):417–433. doi: 10.1085/jgp.83.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982 Jun;38(3):259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Krause H., Kübler M., Herdey A. Kinetics of inactivation and recovery of the slow inward current in the mammalian ventricular myocardium. Pflugers Arch. 1975 Mar 22;355(1):1–17. doi: 10.1007/BF00584795. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976 Dec;263(2):73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Ryanodine prolongs Ca-currents while suppressing contraction in rat ventricular muscle cells. Br J Pharmacol. 1984 Jan;81(1):13–15. doi: 10.1111/j.1476-5381.1984.tb10735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Strontium, nifedipine and 4-aminopyridine modify the time course of the action potential in cells from rat ventricular muscle. Br J Pharmacol. 1984 Mar;81(3):551–556. doi: 10.1111/j.1476-5381.1984.tb10108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R., Trautwein W. The dependence of cardiac contraction on depolarization and slow inward current. Pflugers Arch. 1971;323(3):187–203. doi: 10.1007/BF00586383. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E. Frequency dependence of the ionic currents determining the action potential repolarization in rat ventricular muscle. J Mol Cell Cardiol. 1981 Feb;13(2):207–215. doi: 10.1016/0022-2828(81)90217-0. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y. Parameters affecting the slow inward channel repriming process in frog atrium. J Physiol. 1981 Nov;320:269–291. doi: 10.1113/jphysiol.1981.sp013948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc R Soc Lond B Biol Sci. 1982 Dec 22;217(1206):101–110. doi: 10.1098/rspb.1982.0097. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983 Sep;82(3):385–404. doi: 10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]


