Abstract
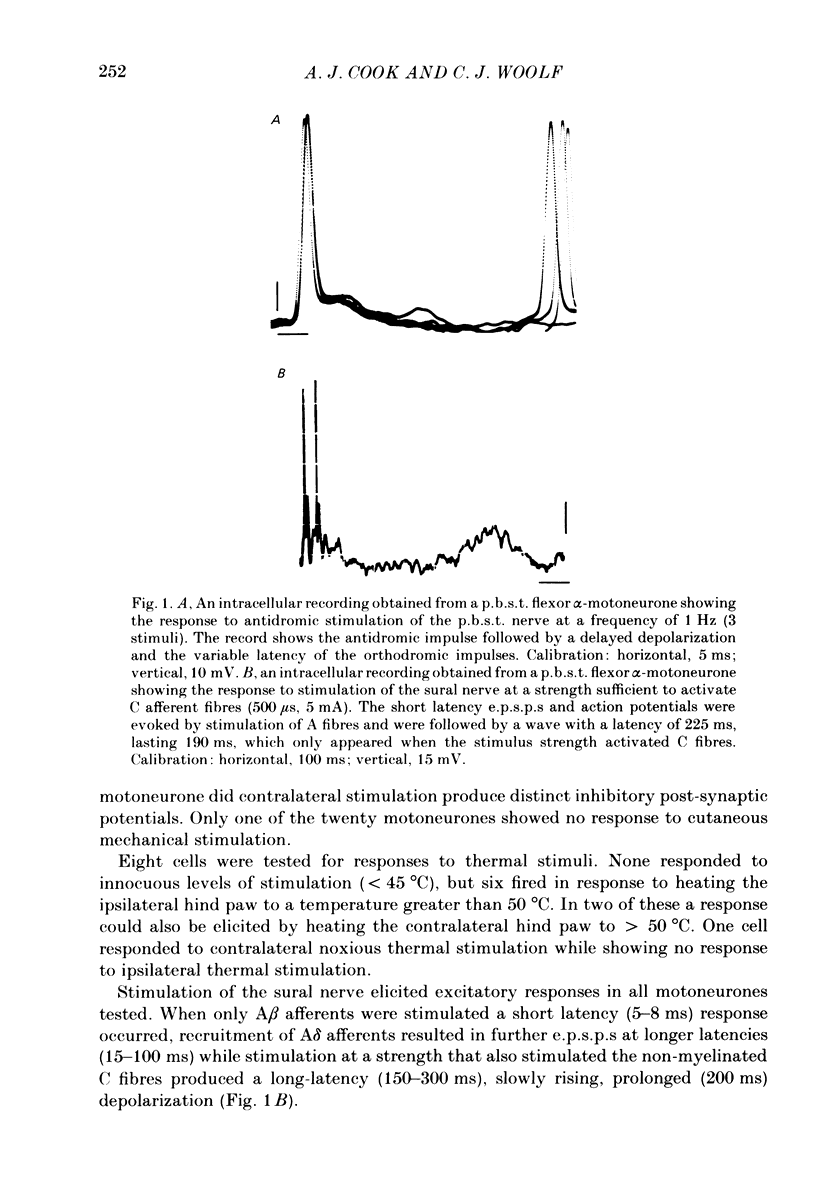
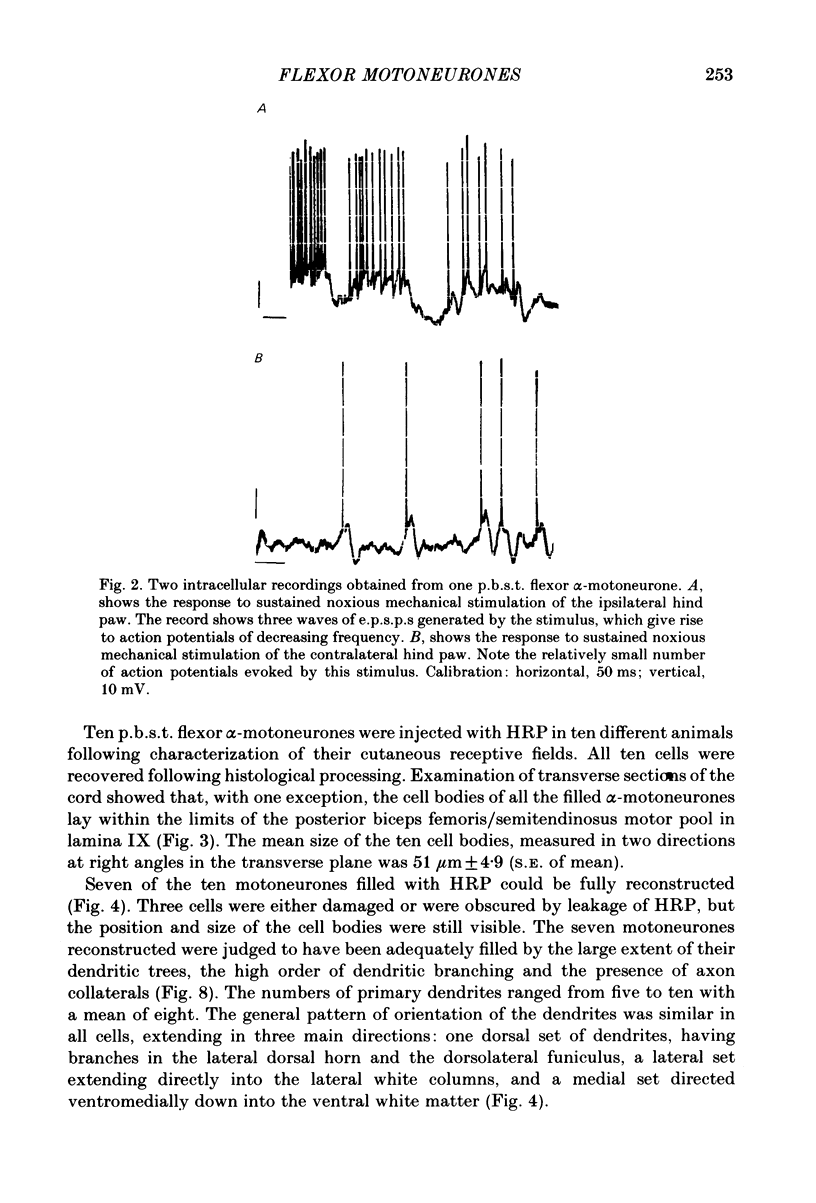
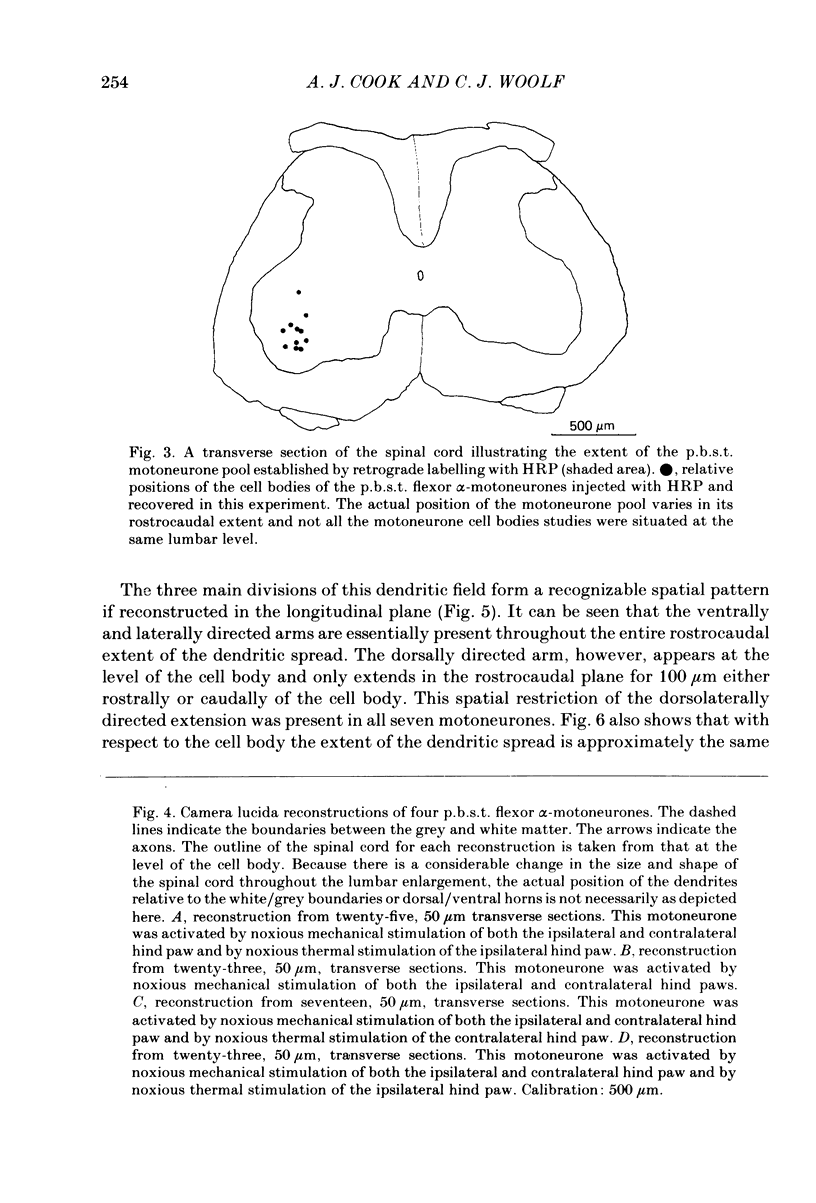
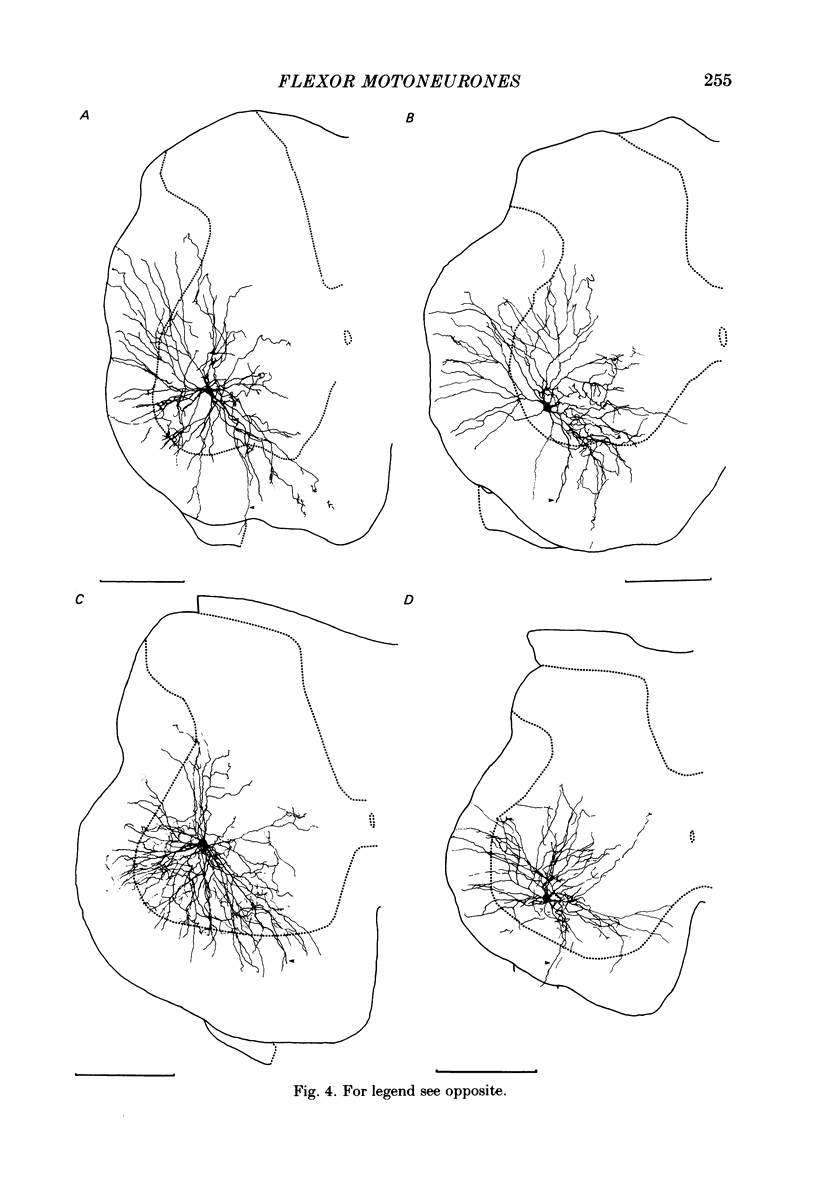
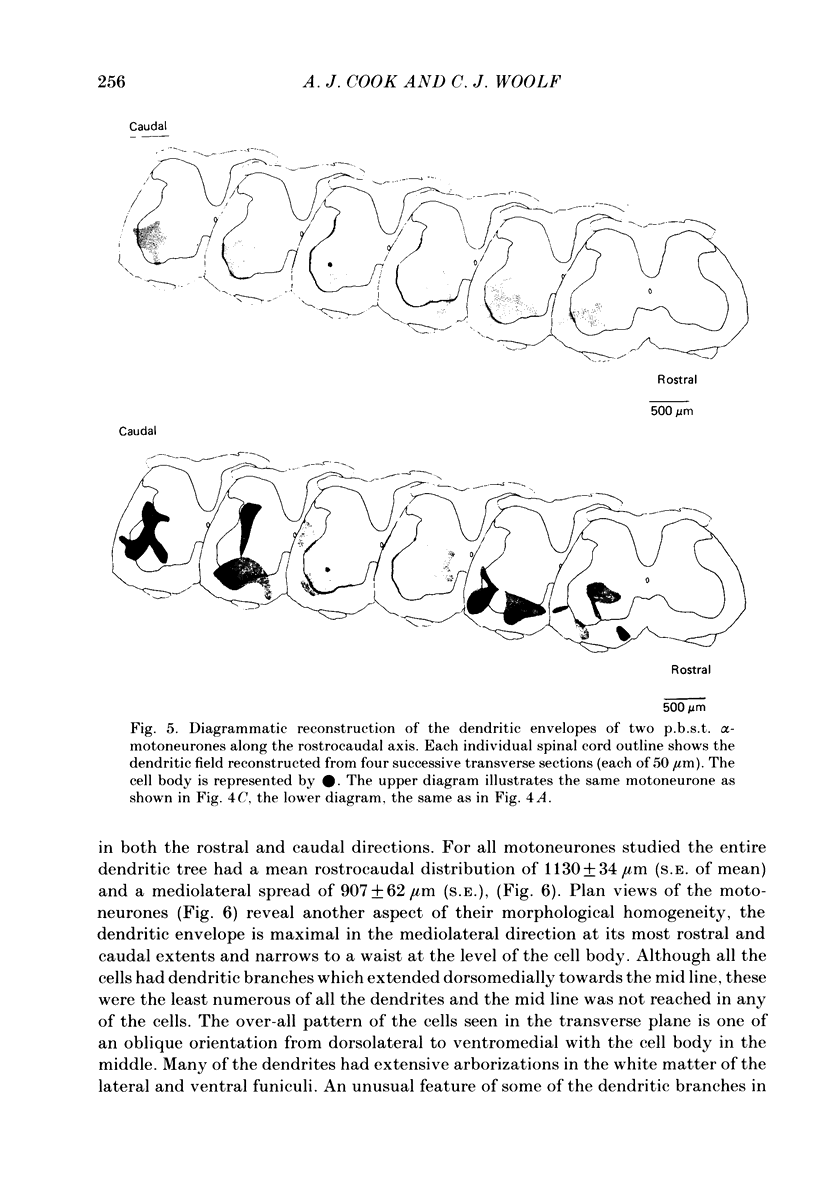
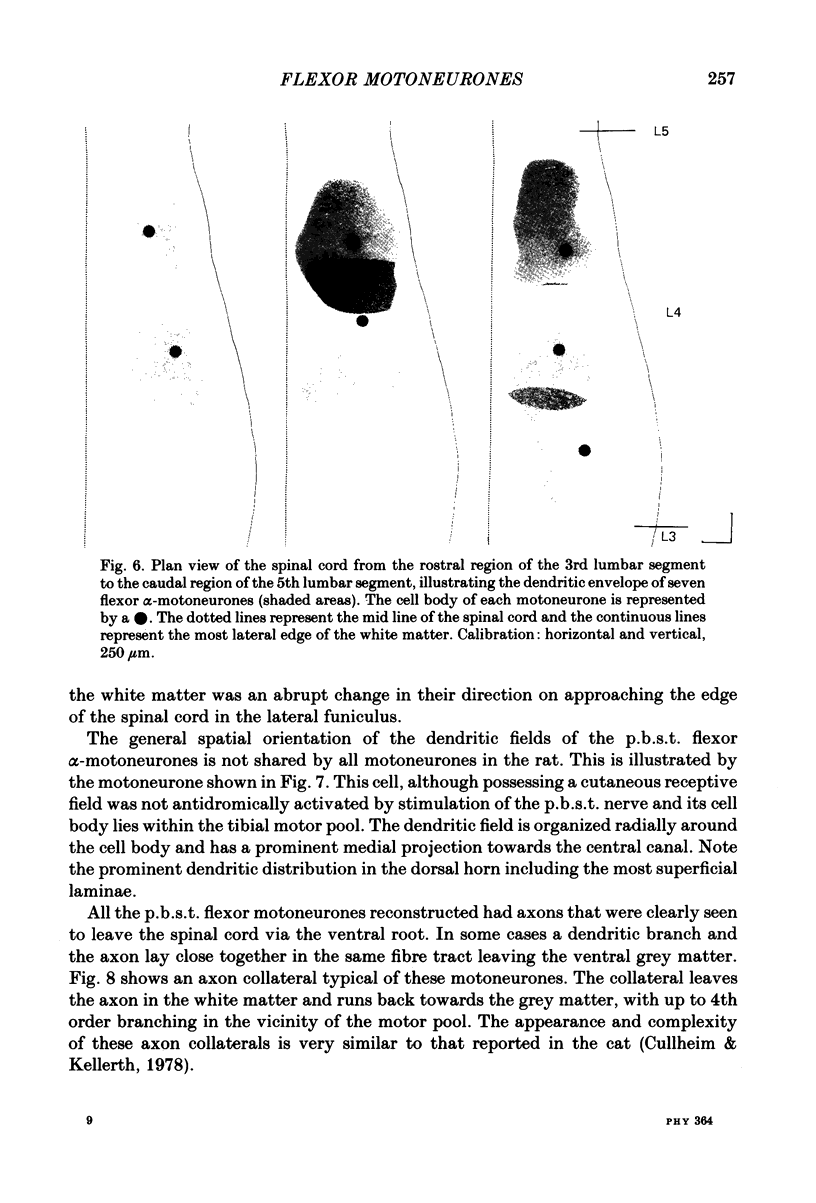
Intracellular recordings have been made from twenty antidromically identified posterior biceps femoris/semitendinosus (p.b.s.t.) hamstring flexor alpha-motoneurones in the decerebrate-spinal rat. The hamstring motoneurones had either low or no spontaneous background activity. In nineteen of the twenty cells high-frequency phasic responses could be elicited by stimulation of the ipsilateral hind paw with firm pressure or pinch. There was no response to light touch or brush. Contralateral cutaneous mechanoreceptive fields with higher thresholds and weaker responses were present in 70% of the motoneurones. Noxious heating of the ipsilateral hind paw produced excitatory responses in six of eight cells tested and two of these cells also responded to heating of the contralateral hind paw. Stimulation of the ipsilateral sural nerve at graded strengths that successively activated A beta, A delta and C afferents produced excitatory post-synaptic potentials (e.p.s.p.s) at progressively longer latencies in the motoneurones. The C-fibre induced e.p.s.p. lasted up to 200 ms. Horseradish peroxidase was injected into ten motoneurones and in seven cases full reconstructions of dendritic field, cell body and axon could be made. In agreement with previous reports from studies in the cat, the dendritic fields of rat motoneurones are very extensive in the rostrocaudal, mediolateral and dorsoventral planes. The general pattern of dendritic branching for each motoneurone in this functionally homogeneous population was uniformly organized. Three major spatial orientations were always present: a rostrocaudally restricted series of dendrites emerging from the cell body and directed dorsolaterally towards the dorsolateral funiculus with branches in the lateral dorsal horn, a laterally, and a ventromedially directed series of branches arranged obliquely in the ventral horn, both of which were distributed rostrocaudally for equal distances from the cell body. Many of these dendritic branches terminated within the lateral and ventral white columns. Although the sizes of the rat flexor motoneurones' somas (51 +/- 4.9 micron, S.E., n = 10) were similar to those of cat lumbosacral alpha-motoneurones, the tip-to-tip rostrocaudal extent of their dendritic fields (1130 +/- 34 micron, S.E., n = 7) was half that reported in the cat. These results are discussed in terms of the organization of the cutaneous flexor withdrawal reflex in the rat.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley K., Somjen G. G. Accommodation in motoneurones of the rat and the cat. J Physiol. 1961 Apr;156(1):75–92. doi: 10.1113/jphysiol.1961.sp006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Harrison P. J., Jankowska E., McCrea D. A., Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983 Oct;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Walmsley B., Hodgson J. A. HRP anatomy of group Ia afferent contacts on alpha motoneurones. Brain Res. 1979 Jan 12;160(2):347–352. doi: 10.1016/0006-8993(79)90430-x. [DOI] [PubMed] [Google Scholar]
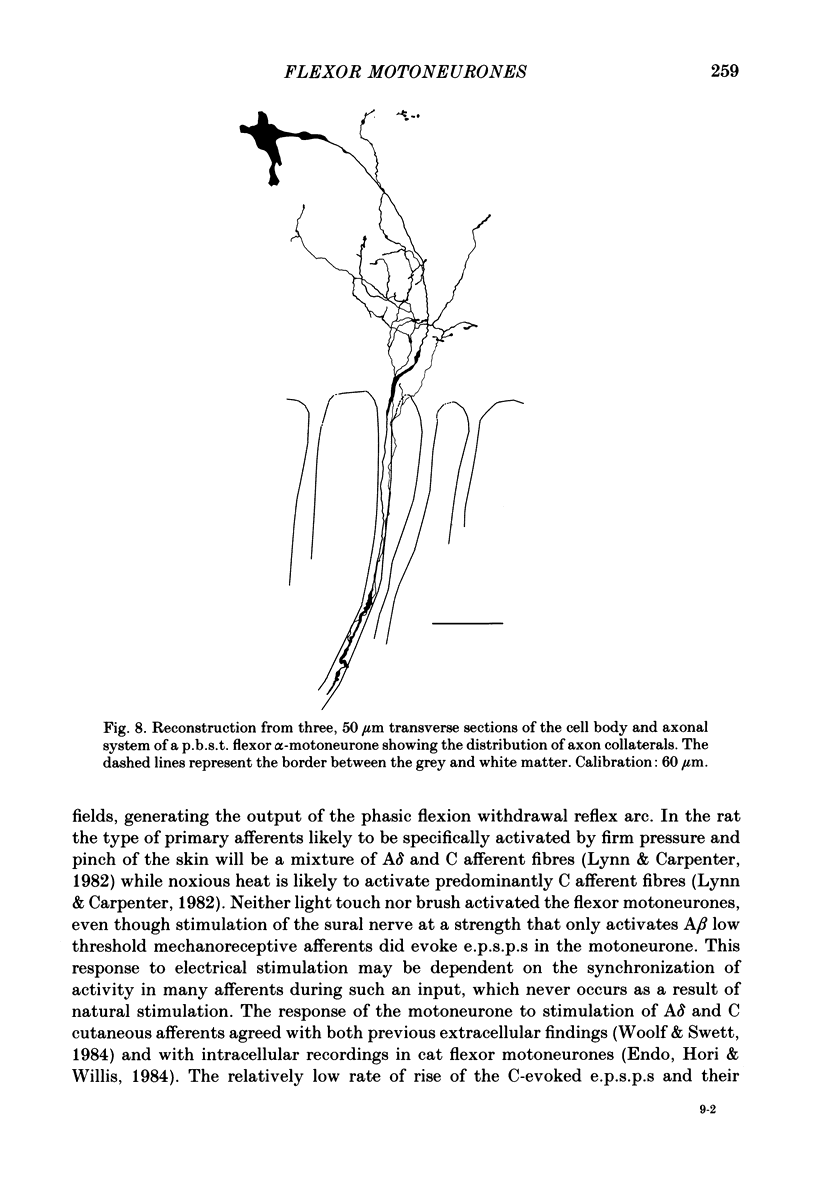
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat sciatic alpha-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1978 Apr 1;178(3):537–557. doi: 10.1002/cne.901780309. [DOI] [PubMed] [Google Scholar]
- Egger M. D., Freeman N. C., Proshansky E. Morphology of spinal motoneurones mediating a cutaneous spinal reflex in the cat. J Physiol. 1980 Sep;306:349–363. doi: 10.1113/jphysiol.1980.sp013401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K., Hori Y., Willis W. D. Postsynaptic potentials evoked in cat hindlimb motoneurons by C-fiber volleys. Neurosci Lett. 1984 Feb 24;44(3):293–298. doi: 10.1016/0304-3940(84)90038-7. [DOI] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SMITH R. S. DELAYED DEPOLARIZATION AND THE REPETITIVE RESPONSE TO INTRACELLULAR STIMULATION OF MAMMALIAN MOTONEURONES. J Physiol. 1963 Oct;168:890–910. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light A. R., Perl E. R. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979 Jul 15;186(2):117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. J Physiol. 1977 Mar;265(3):763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Nicolopoulos-Stournaras S., Iles J. F. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983 Jun 10;217(1):75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett J. E., Woolf C. J. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985 Jan 1;231(1):66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Ulfhake B., Kellerth J. O. A quantitative light microscopic study of the dendrites of cat spinal alpha-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1981 Nov 10;202(4):571–583. doi: 10.1002/cne.902020409. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. A comparison of the structures of alpha and gamma-spinal motoneurones of the cat. J Physiol. 1982 Apr;325:79–91. doi: 10.1113/jphysiol.1982.sp014137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J., Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983 Dec 10;221(3):313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Swett J. E. The cutaneous contribution to the hamstring flexor reflex in the rat: an electrophysiological and anatomical study. Brain Res. 1984 Jun 15;303(2):299–312. doi: 10.1016/0006-8993(84)91216-2. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Wall P. D. Chronic peripheral nerve section diminishes the primary afferent A-fibre mediated inhibition of rat dorsal horn neurones. Brain Res. 1982 Jun 17;242(1):77–85. doi: 10.1016/0006-8993(82)90497-8. [DOI] [PubMed] [Google Scholar]