Abstract
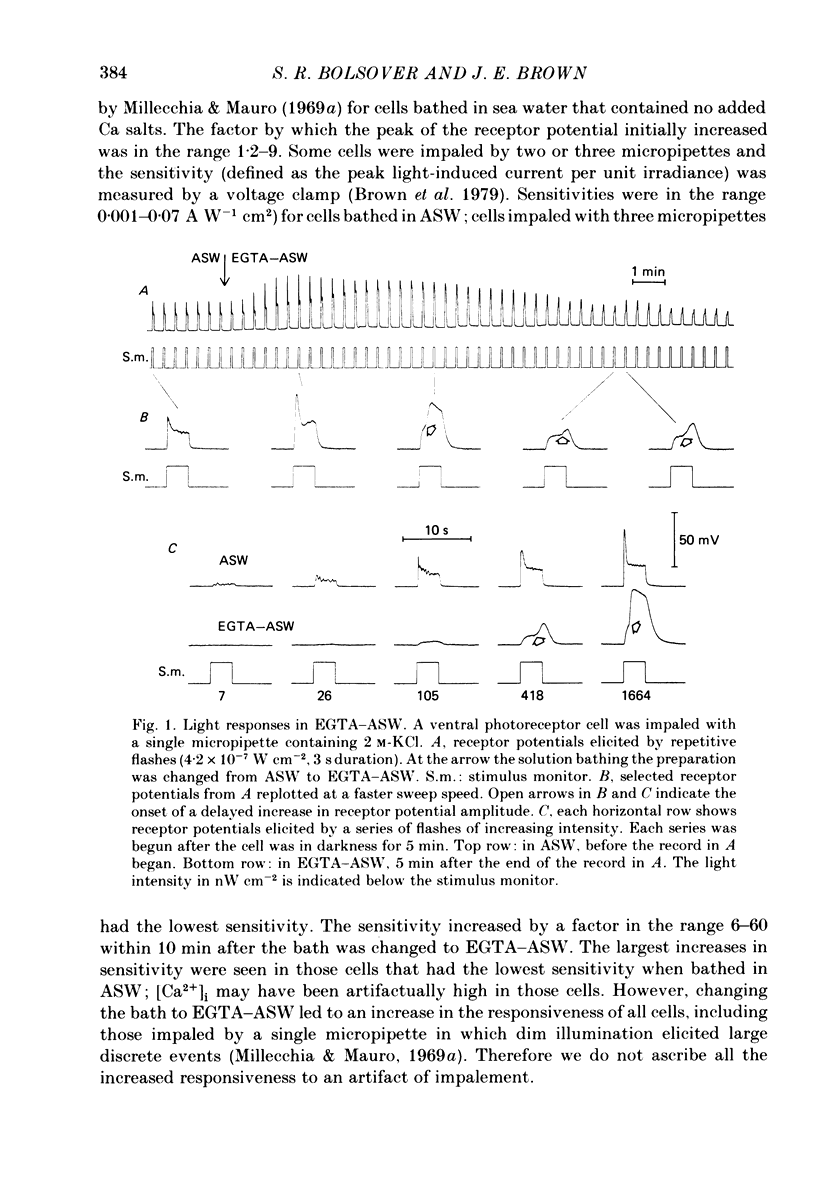
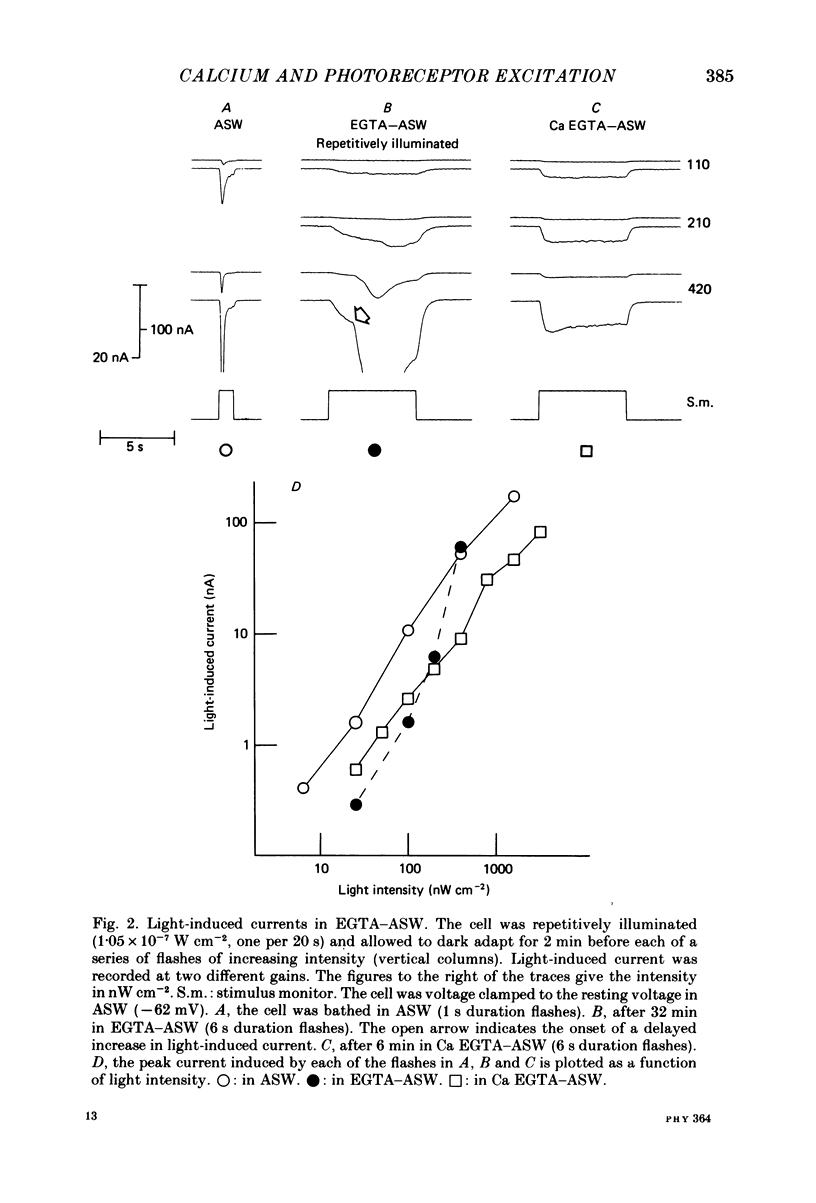
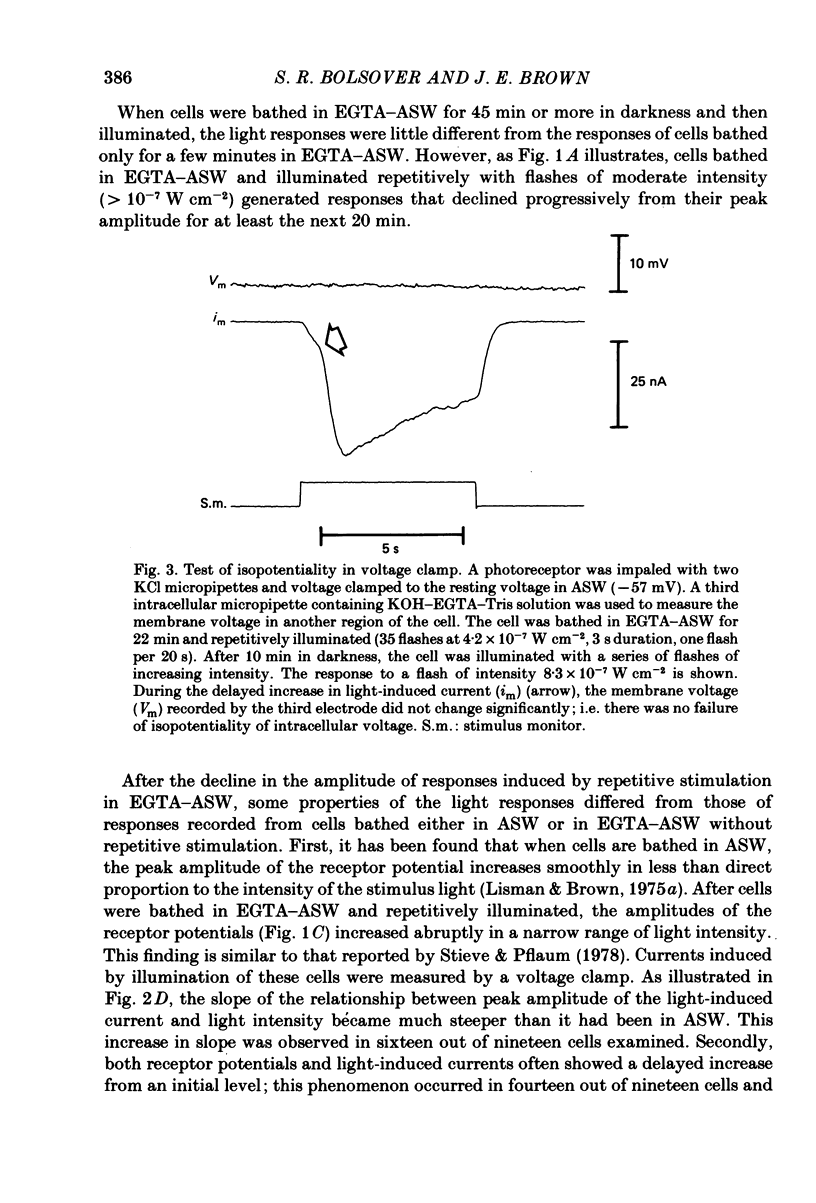
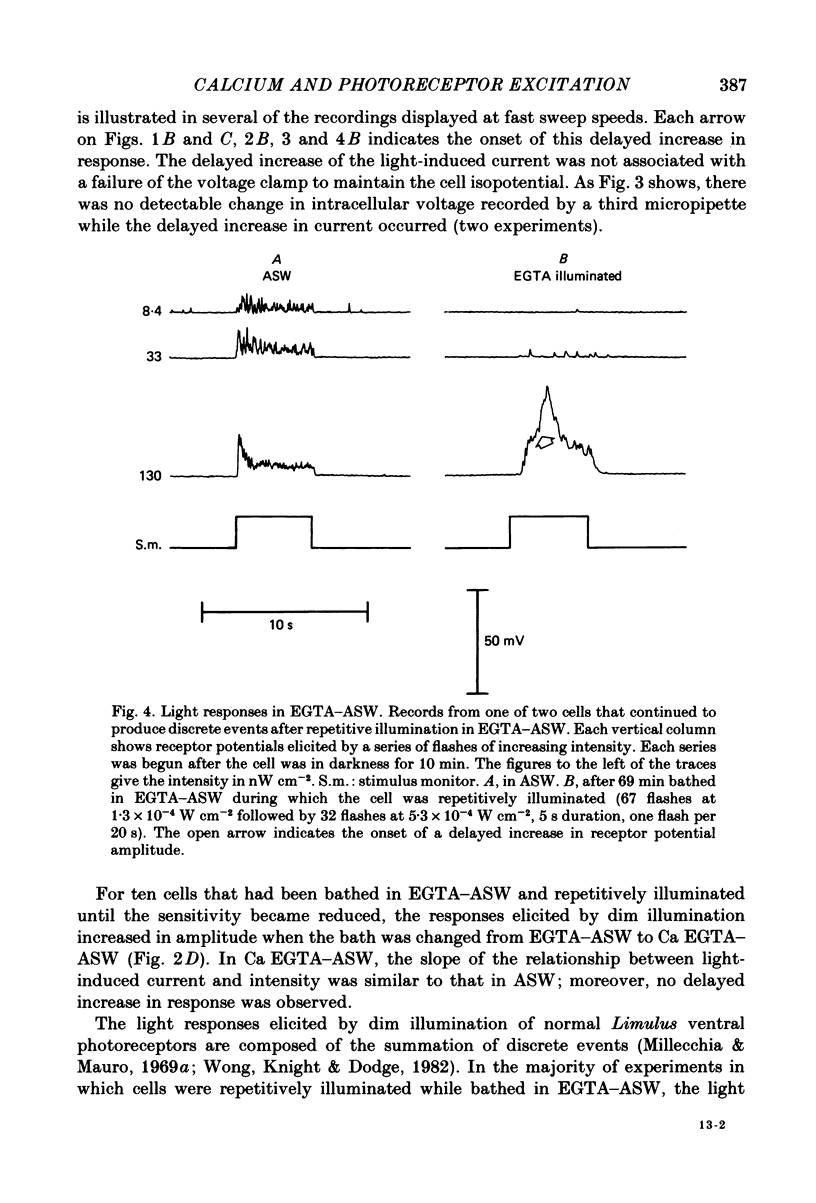
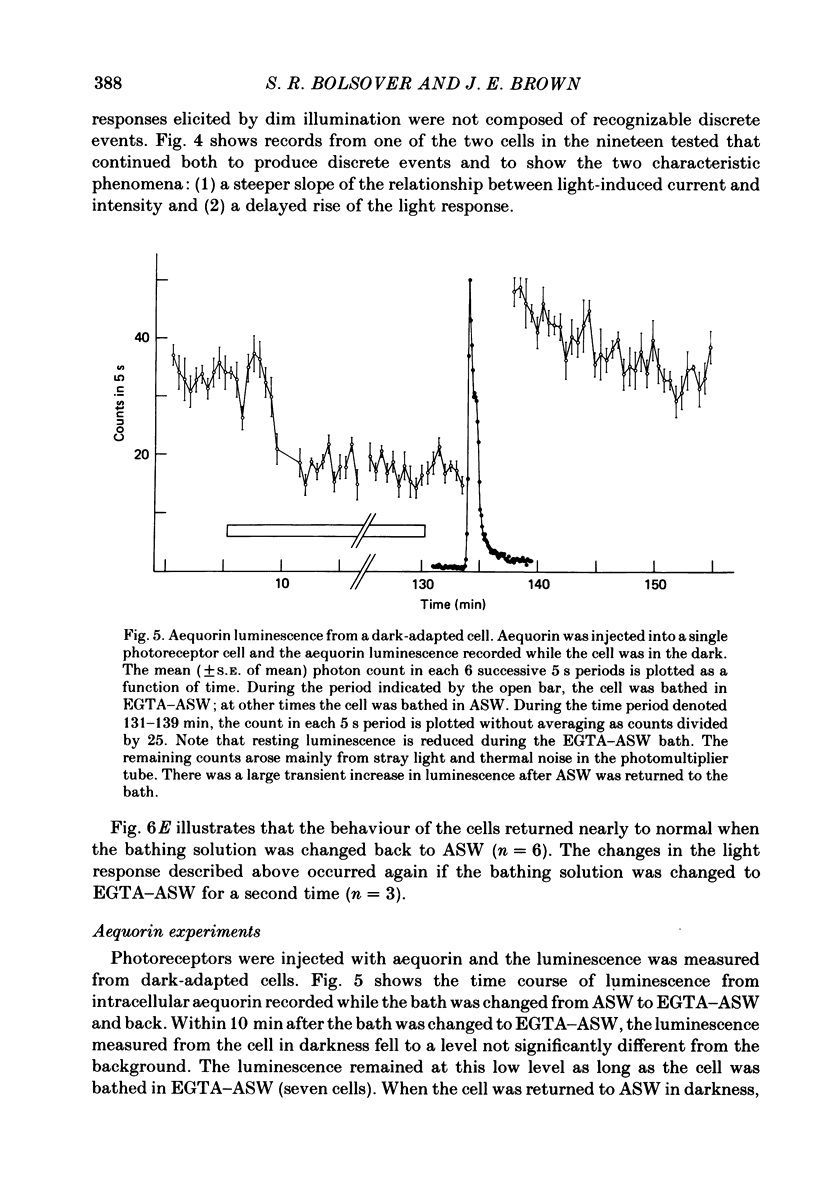
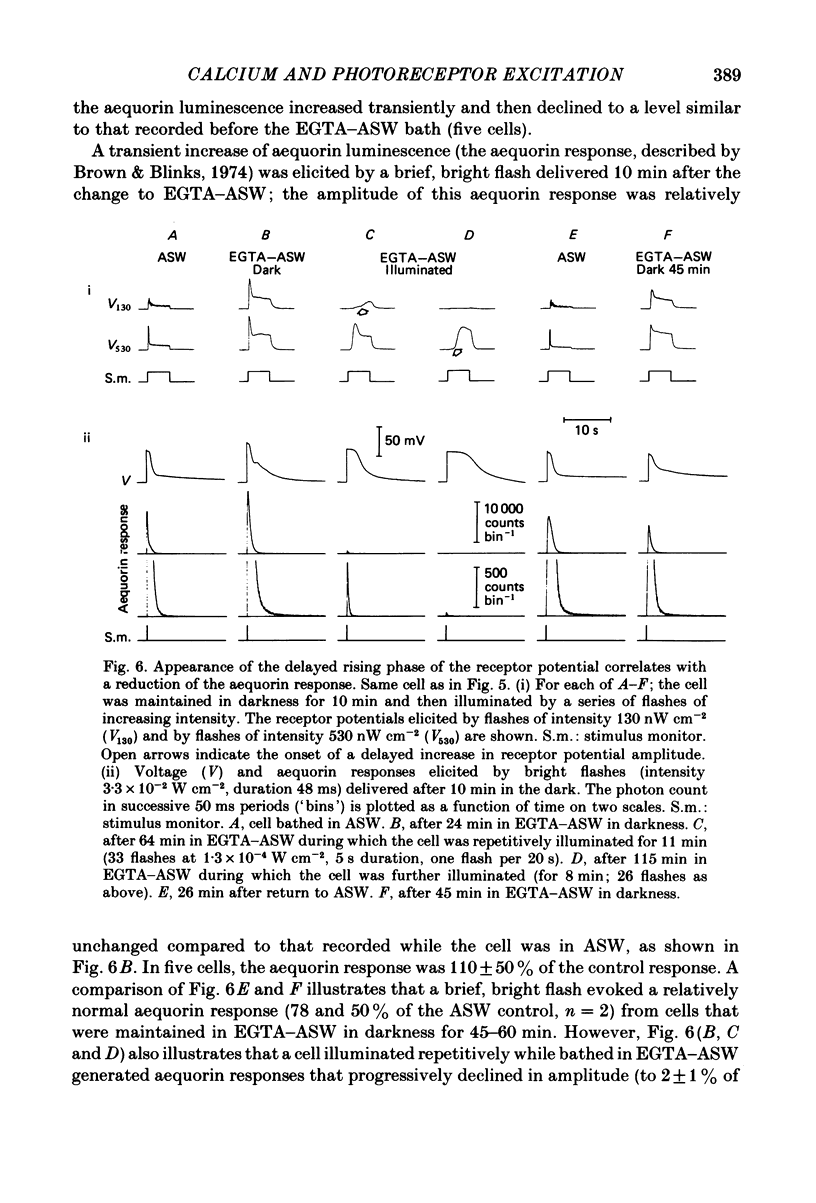
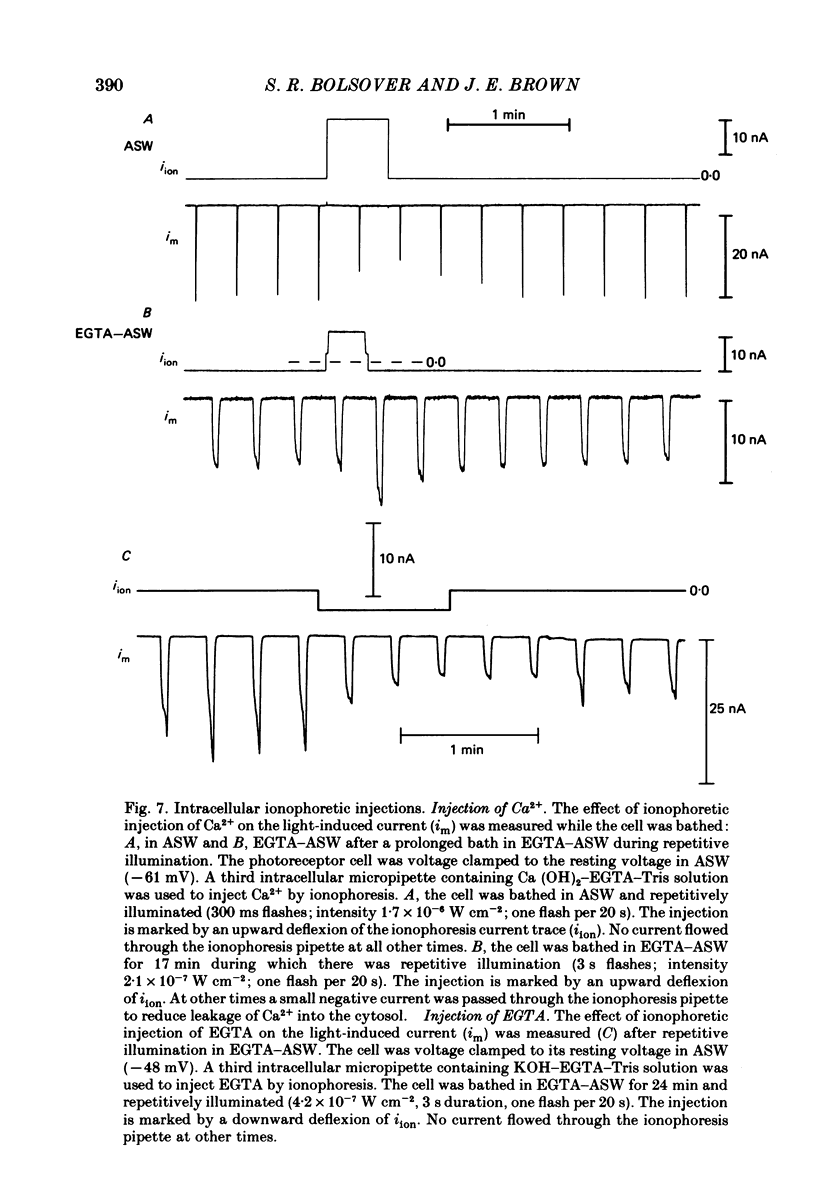
Photoreceptor cells of Limulus ventral eyes were bathed in artificial sea water (ASW) that contained 10 mM-EGTA and no added Ca2+ (EGTA-ASW). Test flashes elicited responses that increased to a maximum size within 10 min in EGTA-ASW but did not change further when dark-adapted cells were bathed for an additional 35 min in this solution. Light responses progressively declined from this maximum size if the cells were repetitively illuminated in EGTA-ASW. In this state of reduced responsiveness, response amplitudes were further reduced by intracellular ionophoretic injection of EGTA; response amplitudes were increased by intracellular ionophoretic injection of Ca2+. Both of these findings are opposite to what is normally observed for cells bathed in ASW. Also, after repetitive illumination in EGTA-ASW, both the slope of the response versus intensity relationship became steeper and light responses often had a delayed increase in amplitude. The light responses and the response versus intensity relation returned to normal when the bathing medium was changed back to ASW containing 10 mM-Ca2+. The light-induced rise in luminescence recorded from photoreceptors injected with the photoprotein aequorin (the 'aequorin response') declined by at most 50% after dark-adapted photoreceptors were bathed in EGTA-ASW for 45 min. However, the aequorin response progressively declined by 98% if cells were repetitively illuminated while bathed in EGTA-ASW. The total intracellular Ca content of whole end-organs was measured by atomic absorption spectroscopy. Total intracellular Ca content did not change significantly while photoreceptors were bathed in EGTA-ASW even after repetitive illumination. We suggest that cytosolic Ca2+ is required by one or more steps in the mechanisms that link rhodopsin isomerization to both (i) an increase in the conductance of the cell membrane to Na+ and (ii) a release of Ca2+ from a light-labile store.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Brown P. K., Pinto L. H. Detection of light-induced changes of intracellular ionized calcium concentration in Limulus ventral photoreceptors using arsenazo III. J Physiol. 1977 May;267(2):299–320. doi: 10.1113/jphysiol.1977.sp011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Harary H. H., Waggoner A. Isopotentiality and an optical determination of series resistance in Limulus ventral photoreceptors. J Physiol. 1979 Nov;296:357–372. doi: 10.1113/jphysiol.1979.sp013010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Charlton J. S. A quantitative comparison of the effects of intracellular calcium injection and light adaptation on the photoresponse of Limulus ventral photoreceptors. J Gen Physiol. 1977 Nov;70(5):591–600. doi: 10.1085/jgp.70.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Lisman J. Localized desensitization of Limulus photoreceptors produced by light or intracellular calcium ion injection. Science. 1975 Mar 21;187(4181):1094–1096. doi: 10.1126/science.1114339. [DOI] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Harary H. H., Brown J. E. Spatially nonuniform changes in intracellular calcium ion concentrations. Science. 1984 Apr 20;224(4646):292–294. doi: 10.1126/science.6710144. [DOI] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Effects of intracellular injection of calcium buffers on light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):489–506. doi: 10.1085/jgp.66.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Light-induced changes of sensitivity in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):473–488. doi: 10.1085/jgp.66.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaz G., Stieve H. The correlation of the receptor potential with the light induced transient increase in intracellular calcium-concentration measured by absorption change of arsenazo III injected into Limulus ventral nerve photoreceptor cell. Biophys Struct Mech. 1980;6(3):191–208. doi: 10.1007/BF00537293. [DOI] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969 Sep;54(3):310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Stieve H., Pflaum M. The response height versus stimulus intensity curve of the ventral nerve photoreceptor of Limulus depending on adaptation and external calcium concentration. Vision Res. 1978;18(6):747–749. doi: 10.1016/0042-6989(78)90155-4. [DOI] [PubMed] [Google Scholar]
- Wong F., Knight B. W., Dodge F. A. Adapting bump model for ventral photoreceptors of Limulus. J Gen Physiol. 1982 Jun;79(6):1089–1113. doi: 10.1085/jgp.79.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]


