Abstract
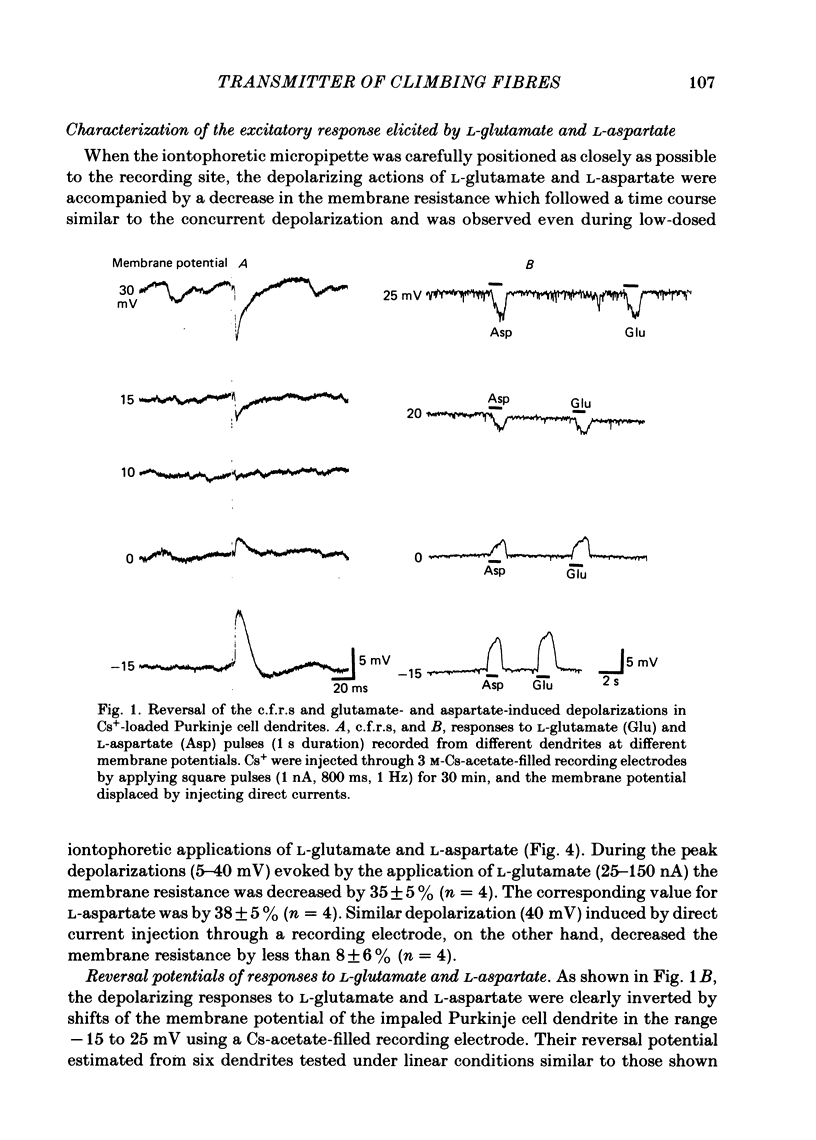
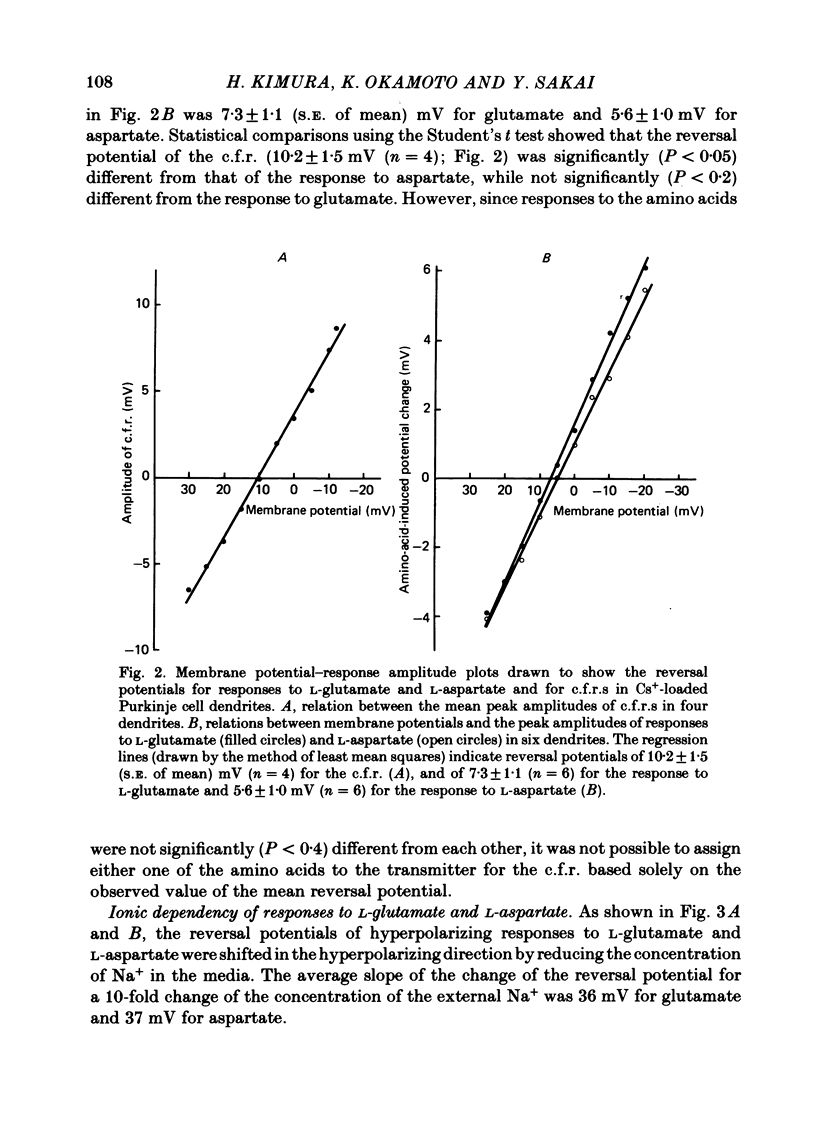
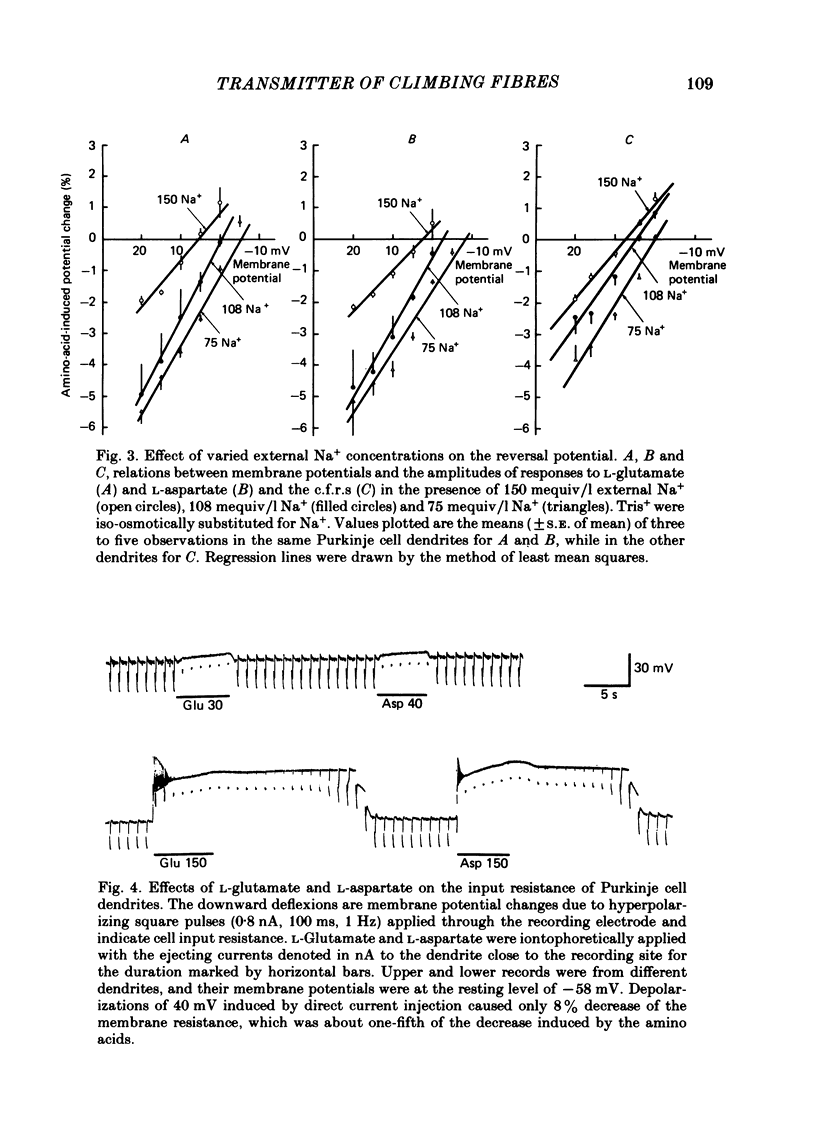
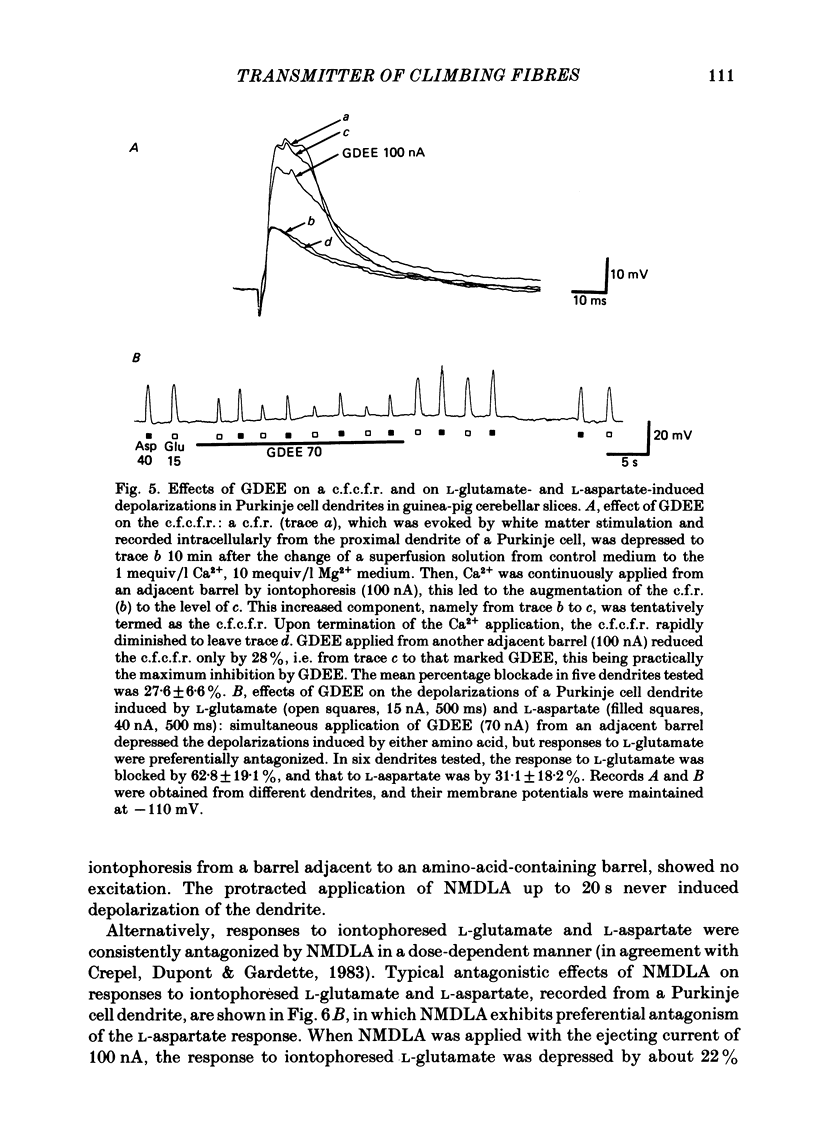
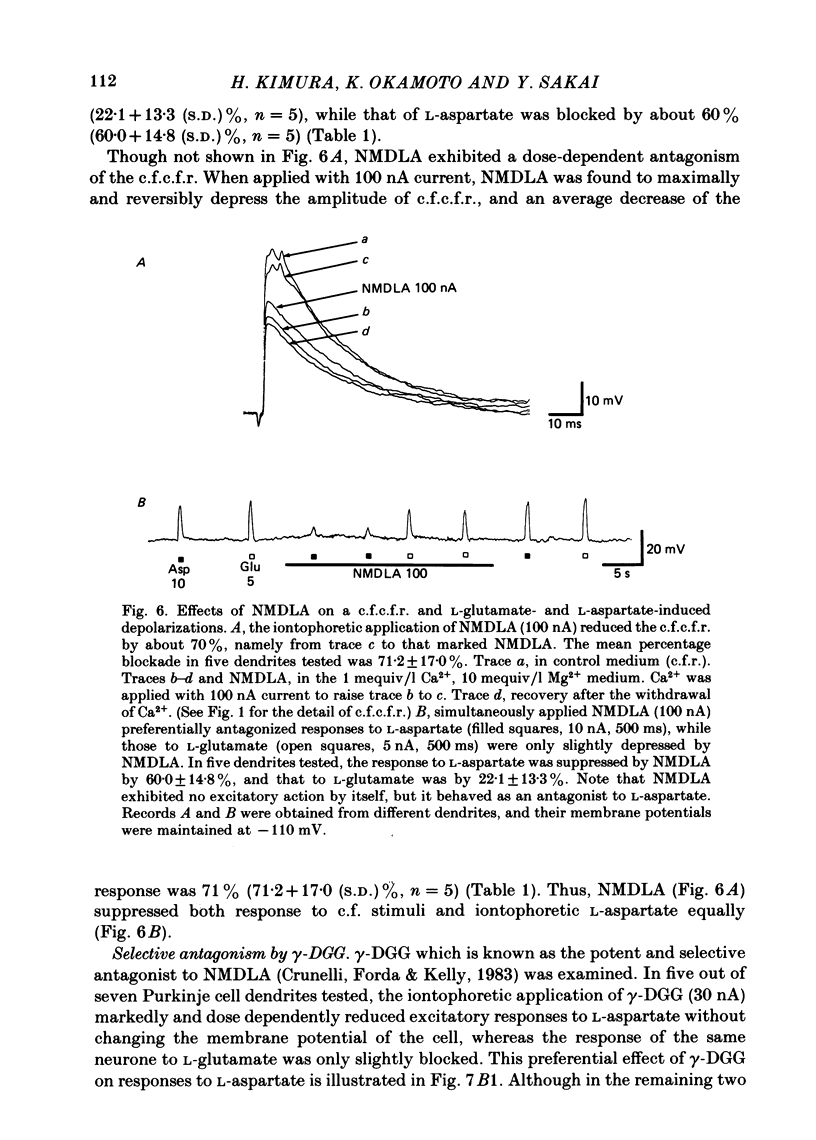
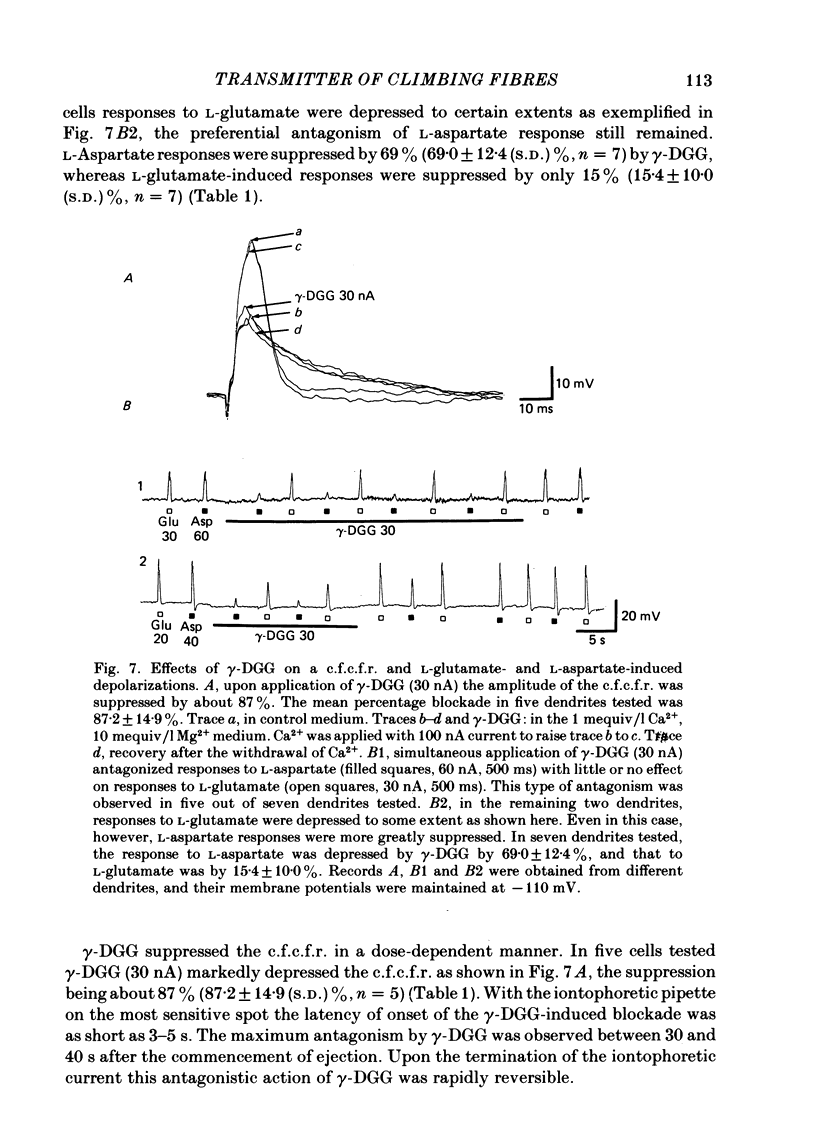
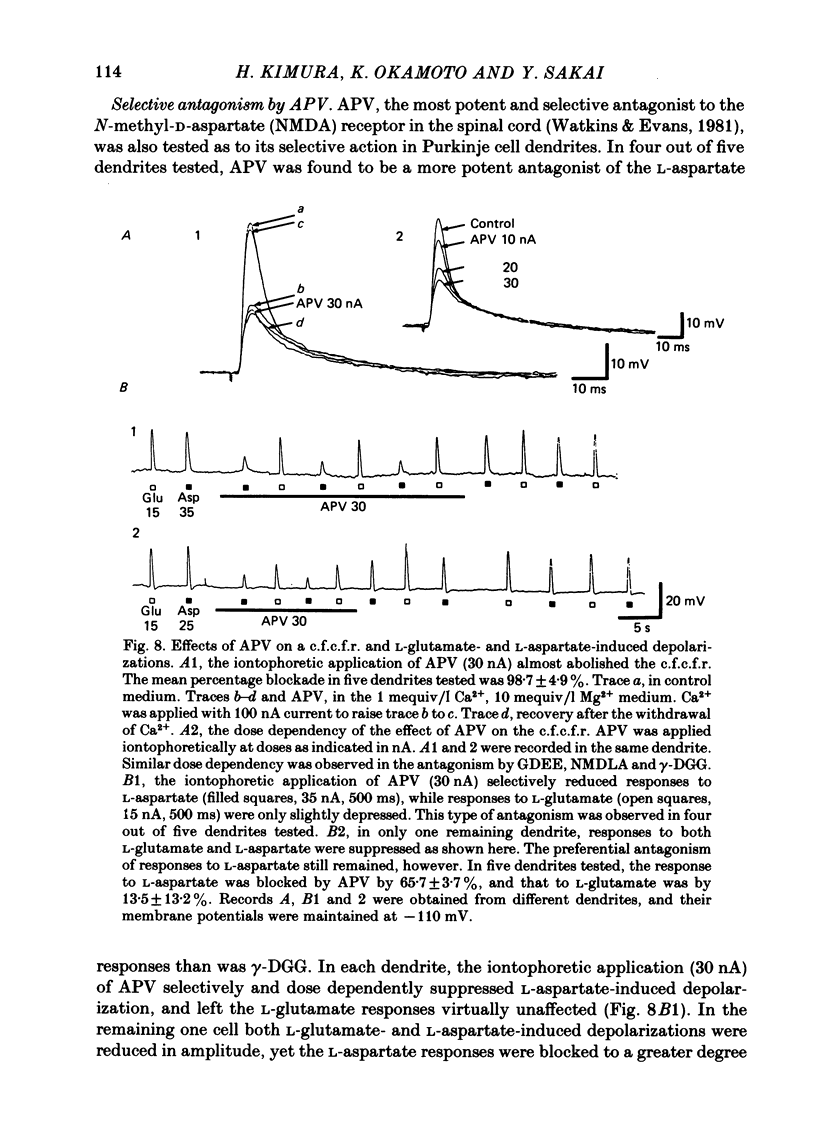
Climbing fibre responses (c.f.r.s) evoked by white matter stimulation and the depolarizations induced by iontophoretically applied L-glutamate and L-aspartate were recorded intracellularly from the proximal dendrites of Purkinje cells in in vitro slice preparations of the guinea-pig cerebellum. Short pulses of L-glutamate and L-aspartate dose-dependently depolarized the Purkinje cell dendrite. Even small doses of these amino acids reduced the input resistance. The maximum decrease in input resistance induced by L-glutamate was 36% and that by L-aspartate was 38%. Intracellular injection of Cs+ allowed Purkinje cell dendrites to be depolarized to a range of -15 to +30 mV. The mean reversal potential for the c.f.r. (Ec) was found to be +10.2 mV (n = 4). The mean reversal potentials obtained for L-glutamate (Eg) and for L-aspartate (Ea) were +7.3 mV (n = 7) and +5.6 mV (n = 7) respectively. When external Na+ concentration was reduced, Ec, Ea and Eg were linearly and similarly shifted in the negative direction, indicating that all these reversal potentials are determined primarily by a Na+ conductance. The effects of the glutamate antagonists 2-amino-5-phosphonovaleric acid (APV), gamma-D-glutamylglycine (gamma-DGG), N-methyl-DL-aspartic acid (NMDLA) and glutamic acid diethylester (GDEE) were compared as to the responses to L-glutamate and L-aspartate and Ca2+-activated focal climbing fibre responses (c.f.c.f.r.s) in order to investigate the receptor type at the synapses formed by the climbing fibres with Purkinje cell dendrites. The order of antagonistic potency to the c.f.c.f.r. was : APV (mean percentage blockade = 99%) greater than gamma-DGG (87%) greater than NMDLA (71%) greater than GDEE (28%). The order of antagonistic potency to the response to L-aspartate was: gamma-DGG (69%) greater than APV (66%) greater than NMDLA (60%) greater than GDEE (31%), and that to the response to L-glutamate was: GDEE (63%) greater than NMDLA (22%) greater than gamma-GDD (15%) greater than APV (14%). APV was found to be the most effective anatagonist of the c.f.c.f.r. Its action was reversible, selective for L-aspartate-induced depolarization and had no effect on the responses to L-glutamate. NMDLA, which has no activity as an agonist, was a greater suppressant of the responses to L-aspartate than those to L-glutamate. These electrophysiological and pharmacological findings suggest that the receptor for the transmitter at the synapses formed by climbing fibres with Purkinje cell dendrites is of the L-aspartate-preferring type, and are thus consistent with the bio-and histochemical findings that L-aspartate may be the endogenous transmitter at this synapse.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collingridge G. L., Kehl S. J., McLennan H. The antagonism of amino acid-induced excitations of rat hippocampal CA1 neurones in vitro. J Physiol. 1983 Jan;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Garthwaite J. Morphological and electrophysiological characteristics of rat cerebellar slices maintained in vitro. J Physiol. 1981 Jul;316:127–138. doi: 10.1113/jphysiol.1981.sp013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dupont J. L., Gardette R. Voltage clamp analysis of the effect of excitatory amino acids and derivatives on Purkinje cell dendrites in rat cerebellar slices maintained in vitro. Brain Res. 1983 Nov 21;279(1-2):311–315. doi: 10.1016/0006-8993(83)90200-7. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. Blockade of amino acid-induced depolarizations and inhibition of excitatory post-synaptic potentials in rat dentate gyrus. J Physiol. 1983 Aug;341:627–640. doi: 10.1113/jphysiol.1983.sp014829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Selective antagonism of amino acid-induced and synaptic excitation in the cat spinal cord. J Physiol. 1979 Dec;297(0):621–635. doi: 10.1113/jphysiol.1979.sp013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. J Physiol. 1983 Oct;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont J. L., Crepel F., Delhaye-Bouchaud N. Influence of bicuculline and picrotoxin on reversal properties of excitatory synaptic potentials in cerebellar Purkinje cells of the rat. Brain Res. 1979 Sep 21;173(3):577–580. doi: 10.1016/0006-8993(79)90256-7. [DOI] [PubMed] [Google Scholar]
- ECCLES J. FUNCTIONAL MEANING OF THE PATTERNS OF SYNAPTIC CONNECTIONS IN THE CEREBELLUM. Perspect Biol Med. 1965;8:289–310. doi: 10.1353/pbm.1965.0041. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K., Voorhoeve P. E. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. J Physiol. 1966 Jan;182(2):297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz J. J., Langmoen I. A. Excitation of hippocampal pyramidal cells by glutamate in the guinea-pig and rat. J Physiol. 1982 Apr;325:317–331. doi: 10.1113/jphysiol.1982.sp014152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J. T., Hou S. M., Cochran S. L. Glutamate and synaptic depolarization of Purkinje cells evoked by parallel fibers and by climbing fibers. Brain Res. 1979 Jul 13;170(2):377–380. doi: 10.1016/0006-8993(79)90118-5. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Hablitz J. J., Wilson W. A. Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature. 1980 Jul 24;286(5771):391–393. doi: 10.1038/286391a0. [DOI] [PubMed] [Google Scholar]
- Langomoen I. A., Hablitz J. J. Reversal potential for glutamate responses in hippocampal pyramidal cells. Neurosci Lett. 1981 Apr 9;23(1):61–65. doi: 10.1016/0304-3940(81)90187-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McLennan H., Haldeman S. The actions of the dimethyl and diethyl esters of glutamic acid on glutamate uptake by brain tissue. J Neurochem. 1973 Feb;20(2):629–631. doi: 10.1111/j.1471-4159.1973.tb12164.x. [DOI] [PubMed] [Google Scholar]
- McLennan H., Lodge D. The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Res. 1979 Jun 15;169(1):83–90. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadi N. S., Kanter D., McBride W. J., Aprison M. H. Effects of 3-acetylpyridine on several putative neurotransmitter amino acids in the cerebellum and medulla of the rat. J Neurochem. 1977 Mar;28(3):661–662. doi: 10.1111/j.1471-4159.1977.tb10439.x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Kimura H., Sakai Y. Ionic mechanisms of the action of taurine on cerebellar Purkinje cell dendrites in vitro: intradendritic study. Brain Res. 1983 Feb 7;260(2):261–269. doi: 10.1016/0006-8993(83)90679-0. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Permeability changes produced by L-glutamate at the excitatory post-synaptic membrane of the crayfish muscle. J Physiol. 1976 Mar;255(3):669–685. doi: 10.1113/jphysiol.1976.sp011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval M. E., Cotman C. W. Evaluation of glutamate as a neurotransmitter of cerebellar parallel fibers. Neuroscience. 1978;3(2):199–206. doi: 10.1016/0306-4522(78)90101-x. [DOI] [PubMed] [Google Scholar]
- Sawada S., Takada S., Yamamoto C. Selective activation of synapses near the tip of drug-ejecting microelectrode, and effects of antagonists of excitatory amino acids in the hippocampus. Brain Res. 1983 May 9;267(1):156–160. doi: 10.1016/0006-8993(83)91050-8. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Velumian A. A. Mechanisms of post-synaptic excitation in amphibian motoneurones. J Physiol. 1978 Jun;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Glutamate as the neurotransmitter of cerebellar granule cells in the rat: electrophysiological evidence. Br J Pharmacol. 1979 Jun;66(2):291–296. doi: 10.1111/j.1476-5381.1979.tb13678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wiklund L., Toggenburger G., Cuénod M. Aspartate: possible neurotransmitter in cerebellar climbing fibers. Science. 1982 Apr 2;216(4541):78–80. doi: 10.1126/science.6121375. [DOI] [PubMed] [Google Scholar]


