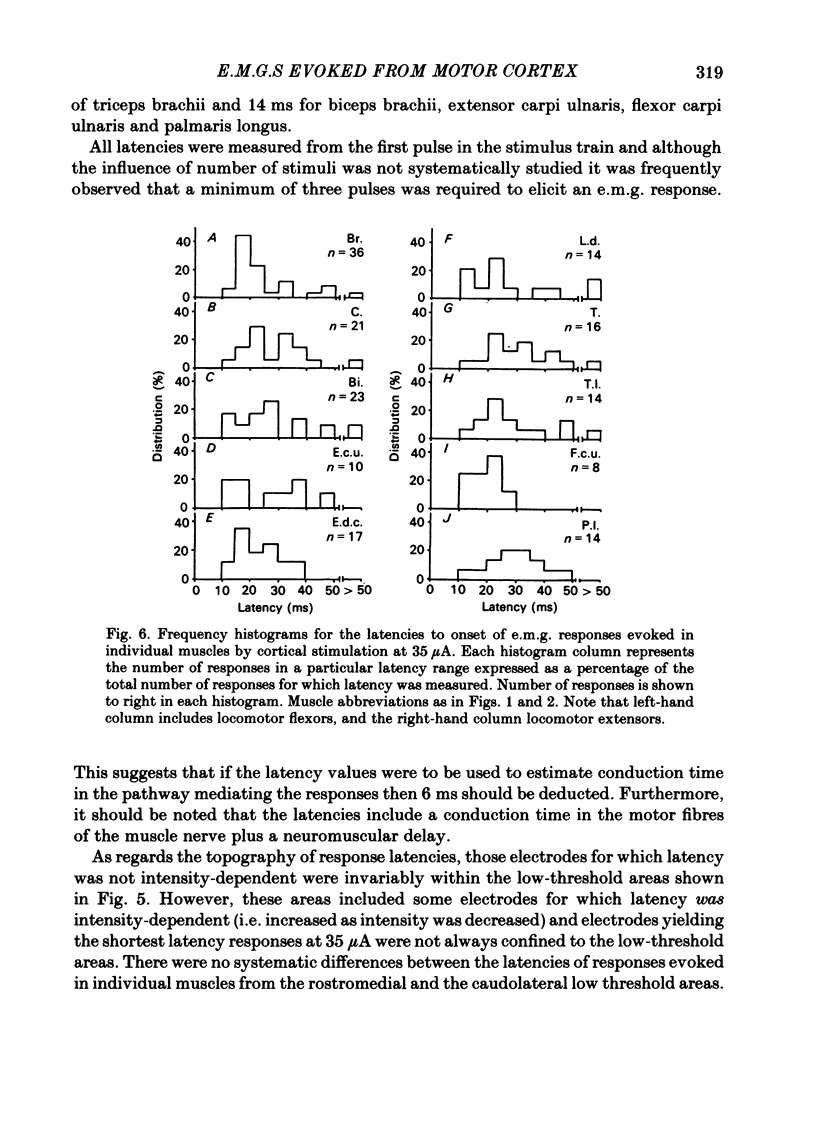
Abstract
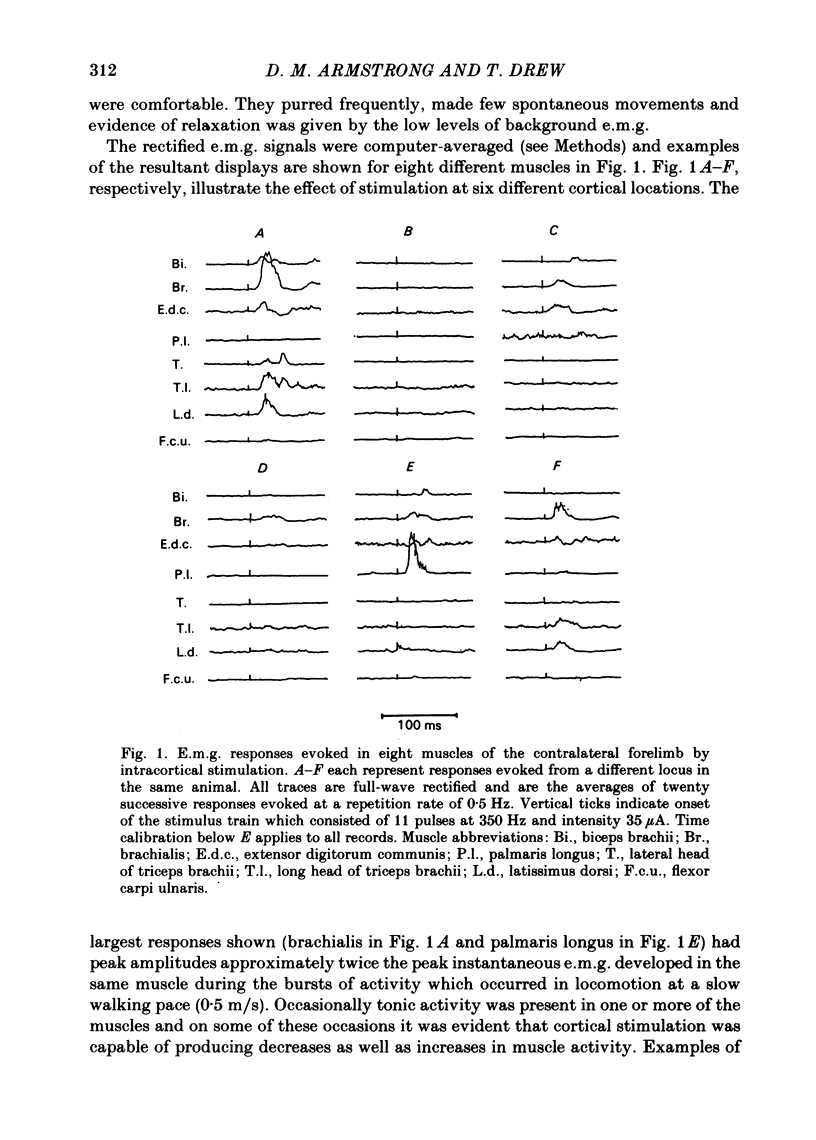
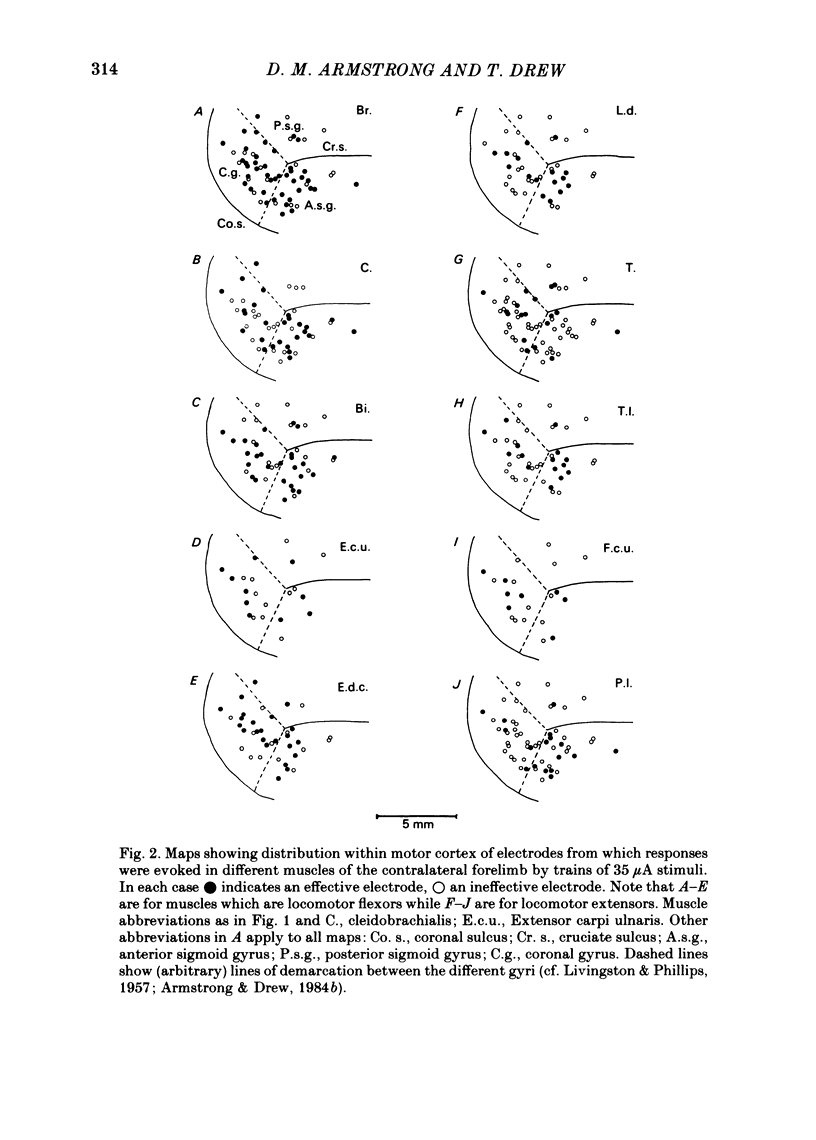
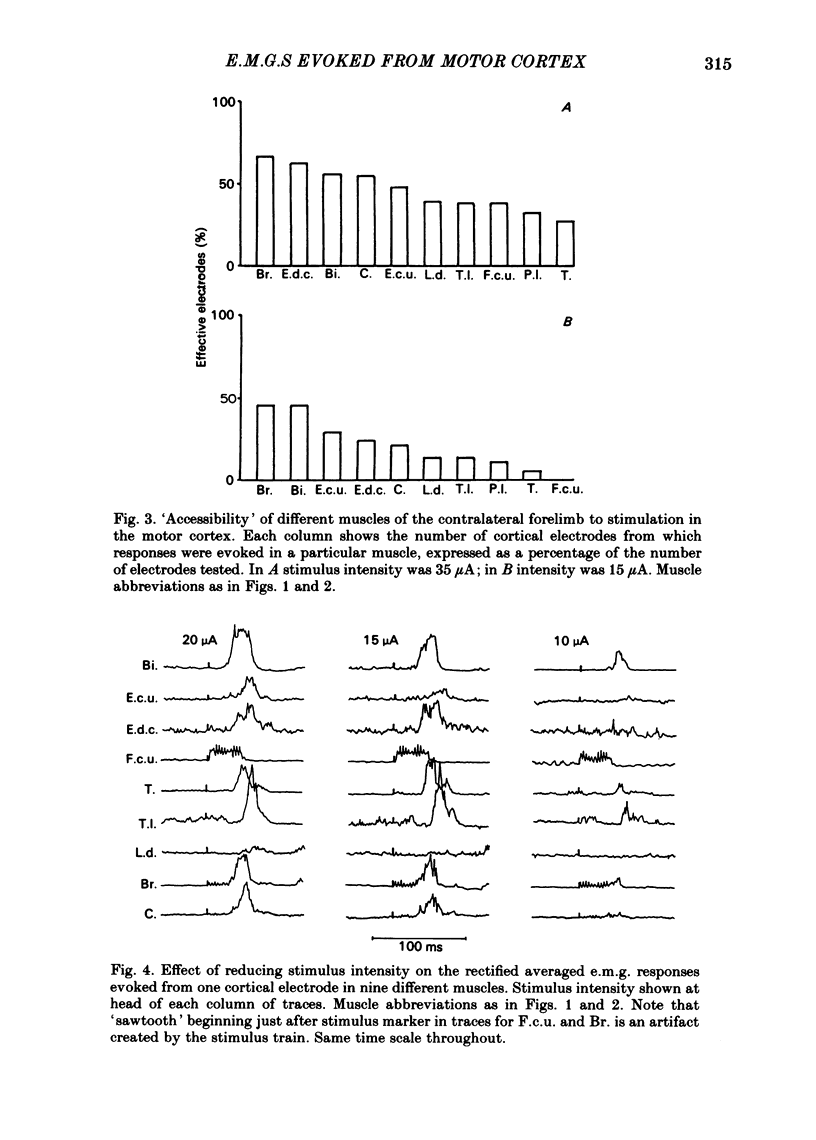
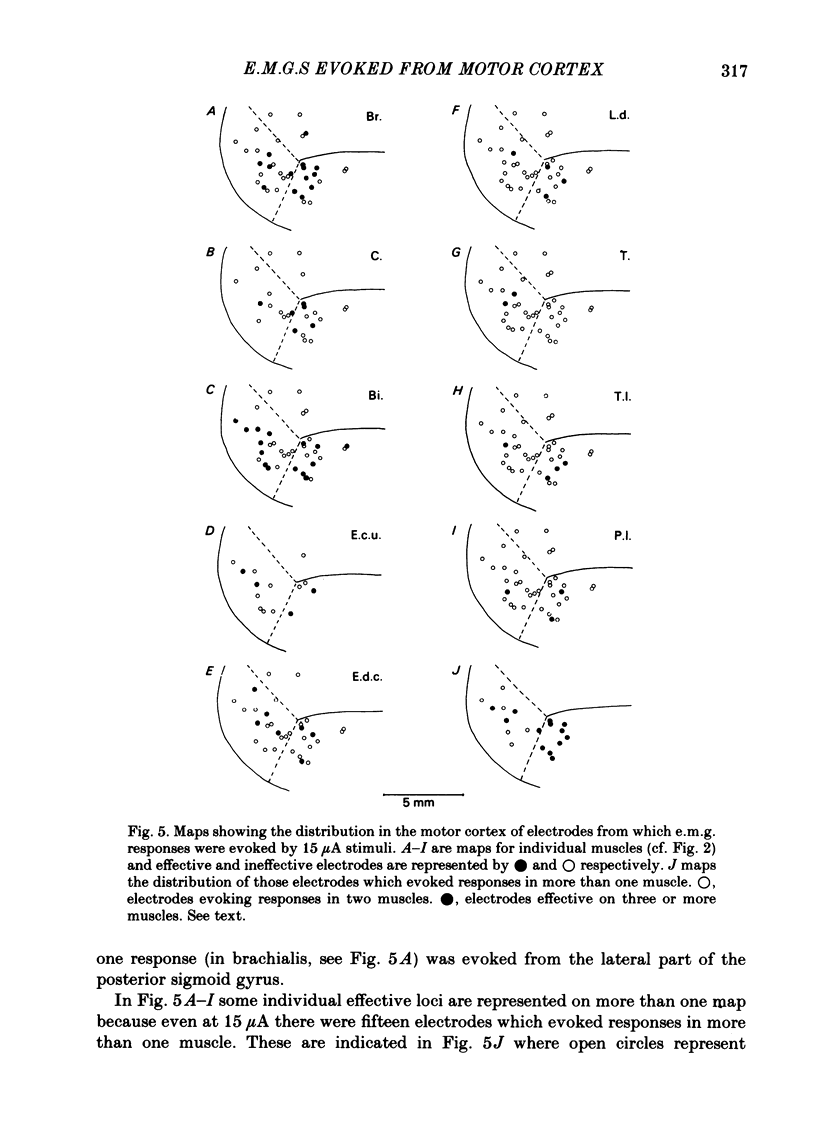
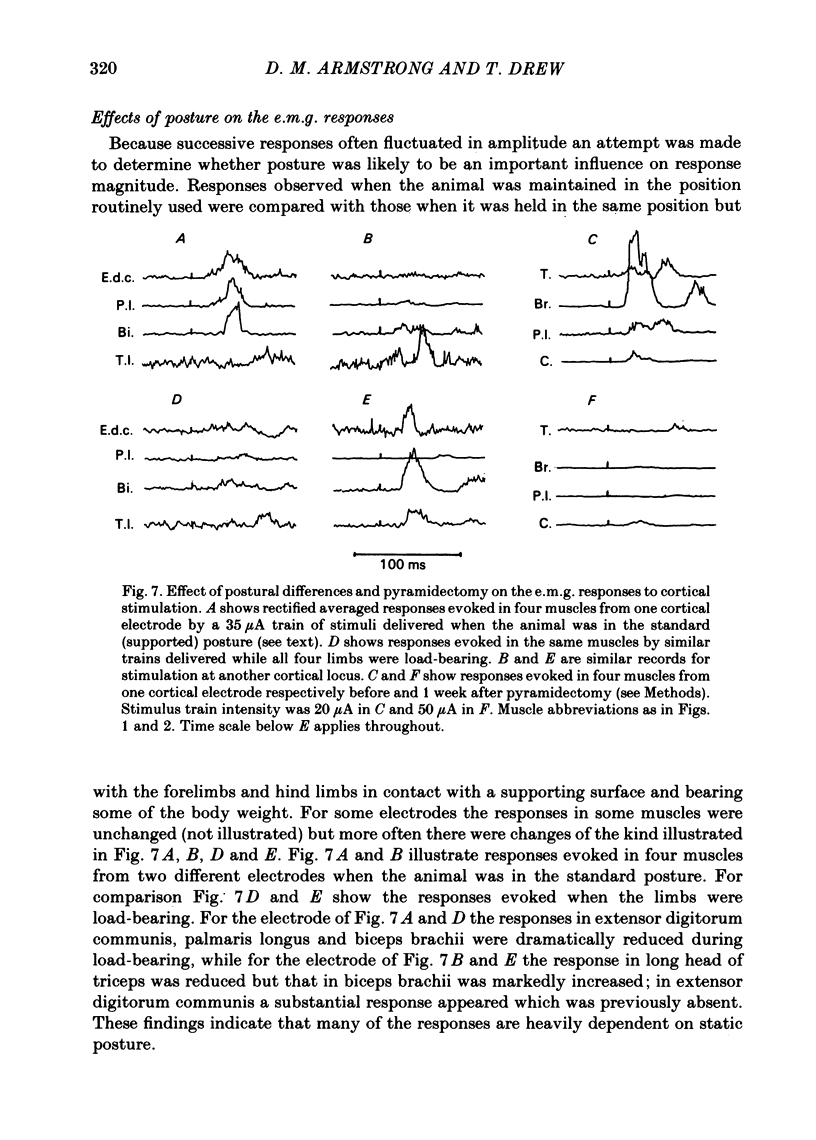
Chronically implanted microwires were used to deliver brief trains of electrical stimuli (11 cathodal pulses at 330 Hz and intensity 5-35 microA) to sixty-two locations in the grey matter of the pericruciate cortex in cats. Electromyographic (e.m.g.) responses in the contralateral forelimb were recorded from a total of ten muscles (four to eight in each animal) acting about the shoulder, elbow and wrist and on the digits. The animals were relaxed with little background e.m.g. in the muscles and as a result only excitatory effects could be described. Five muscles which are flexors in the locomotor context were excited from more electrodes, distributed more widely across the motor cortex, than another five muscles which are extensors during locomotion; this difference in 'accessibility' was present both at 35 microA stimulus intensity and at 15 microA. At a stimulus intensity of 15 microA, effective cortical electrodes tended to cluster either in the most lateral part of the anterior sigmoid gyrus (rostromedial focus) or in the coronal gyrus just caudal to a line prolonged beyond the lateral end of the cruciate sulcus (caudolateral focus). This is consistent with the existence of a double motor representation within the forelimb motor cortex (Pappas & Strick, 1981). The two foci were similar in that both gave rise to more flexor than extensor responses and to fewer responses in digit or wrist muscles than in muscles acting about more proximal joints (elbow and shoulder). At stimulus intensity 35 microA the latency of the earliest e.m.g. responses ranged from 11 to 14 ms in different muscles. For some muscles and electrodes the amplitude of the e.m.g. responses was substantially altered by a quite small postural change. After pyramidectomy the cortical thresholds and the e.m.g. latencies were both greatly increased.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins R. J., Cegnar M. R., Rafuse D. D. Differential effects of lesions of the anterior and posterior sigmoid gyri in cats. Brain Res. 1971 Jul 23;30(2):411–414. doi: 10.1016/0006-8993(71)90092-8. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 1984 Jan;346:471–495. doi: 10.1113/jphysiol.1984.sp015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Forelimb electromyographic responses to motor cortex stimulation during locomotion in the cat. J Physiol. 1985 Oct;367:327–351. doi: 10.1113/jphysiol.1985.sp015827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Drew T. Topographical localization in the motor cortex of the cat for somatic afferent responses and evoked movements. J Physiol. 1984 May;350:33–54. doi: 10.1113/jphysiol.1984.sp015187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H., Arnold A., Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res. 1976 Dec 22;26(5):443–461. doi: 10.1007/BF00238820. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Stoney S. D., Jr, Abzug C. Relationship between afferent input and motor outflow in cat motorsensory cortex. J Neurophysiol. 1968 Sep;31(5):670–681. doi: 10.1152/jn.1968.31.5.670. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Stoney S. D., Jr, Thompson W. D. Characteristics of cervical interneurones which mediate cortical motor outflow to distal forelimb muscles of cats. Brain Res. 1971 Mar 19;27(1):79–95. doi: 10.1016/0006-8993(71)90373-8. [DOI] [PubMed] [Google Scholar]
- Futami T., Shinoda Y., Yokota J. Spinal axon collaterals of corticospinal neurons identified by intracellular injection of horseradish peroxidase. Brain Res. 1979 Mar 23;164:279–284. doi: 10.1016/0006-8993(79)90021-0. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Padel Y., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3--C4 propriospinal neurones. Exp Brain Res. 1978 Sep 15;33(1):101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 1. Pyramidal effects on motoneurones. Exp Brain Res. 1976 Dec 22;26(5):509–519. doi: 10.1007/BF00238824. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriospinal neurones transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp Brain Res. 1977 Sep 28;29(3-4):323–346. doi: 10.1007/BF00236174. [DOI] [PubMed] [Google Scholar]
- LIVINGSTON A., PHILLIPS C. G. Maps and thresholds for the sensorimotor cortex of the cat. Q J Exp Physiol Cogn Med Sci. 1957 Apr;42(2):190–205. doi: 10.1113/expphysiol.1957.sp001250. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Rispal-Padel L. Somatotopic localization in cat motor cortex. Brain Res. 1976 Apr 9;105(3):405–422. doi: 10.1016/0006-8993(76)90590-4. [DOI] [PubMed] [Google Scholar]
- Pappas C. L., Strick P. L. Physiological demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol. 1981 Aug 20;200(4):481–490. doi: 10.1002/cne.902000403. [DOI] [PubMed] [Google Scholar]
- Sakata H., Miyamoto J. Topographic relationship between the receptive fields of neurons in the motor cortex and the movements elicited by focal stimulation in freely moving cats. Jpn J Physiol. 1968 Aug 15;18(4):489–507. doi: 10.2170/jjphysiol.18.489. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Arnold A. P., Asanuma H. Spinal branching of corticospinal axons in the cat. Exp Brain Res. 1976 Oct 28;26(3):215–234. doi: 10.1007/BF00234928. [DOI] [PubMed] [Google Scholar]
- Shinoda Y., Yamaguchi T. The intraspinal branching patterns of fast and slow pyramidal tract neurons in the cat. J Physiol (Paris) 1978;74(3):237–238. [PubMed] [Google Scholar]
- Strick P. L., Preston J. B. Multiple representation in the primate motor cortex. Brain Res. 1978 Oct 13;154(2):366–370. doi: 10.1016/0006-8993(78)90707-2. [DOI] [PubMed] [Google Scholar]
- Strick P. L., Preston J. B. Sorting of somatosensory afferent information in primate motor cortex. Brain Res. 1978 Nov 10;156(2):364–368. doi: 10.1016/0006-8993(78)90520-6. [DOI] [PubMed] [Google Scholar]
- Tanji J., Wise S. P. Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol. 1981 Mar;45(3):467–481. doi: 10.1152/jn.1981.45.3.467. [DOI] [PubMed] [Google Scholar]
- Yumiya H., Ghez C. Specialized subregions in the cat motor cortex: anatomical demonstration of differential projections to rostral and caudal sectors. Exp Brain Res. 1984;53(2):259–276. doi: 10.1007/BF00238155. [DOI] [PubMed] [Google Scholar]


