Abstract
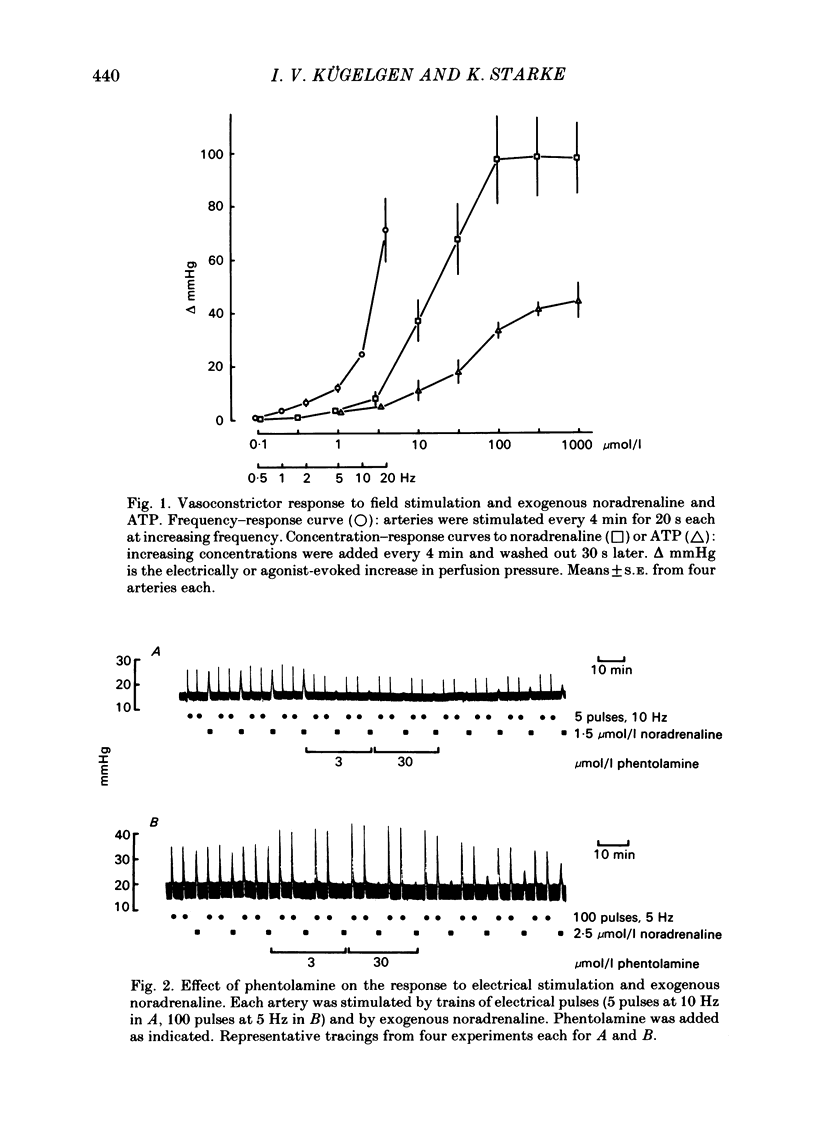
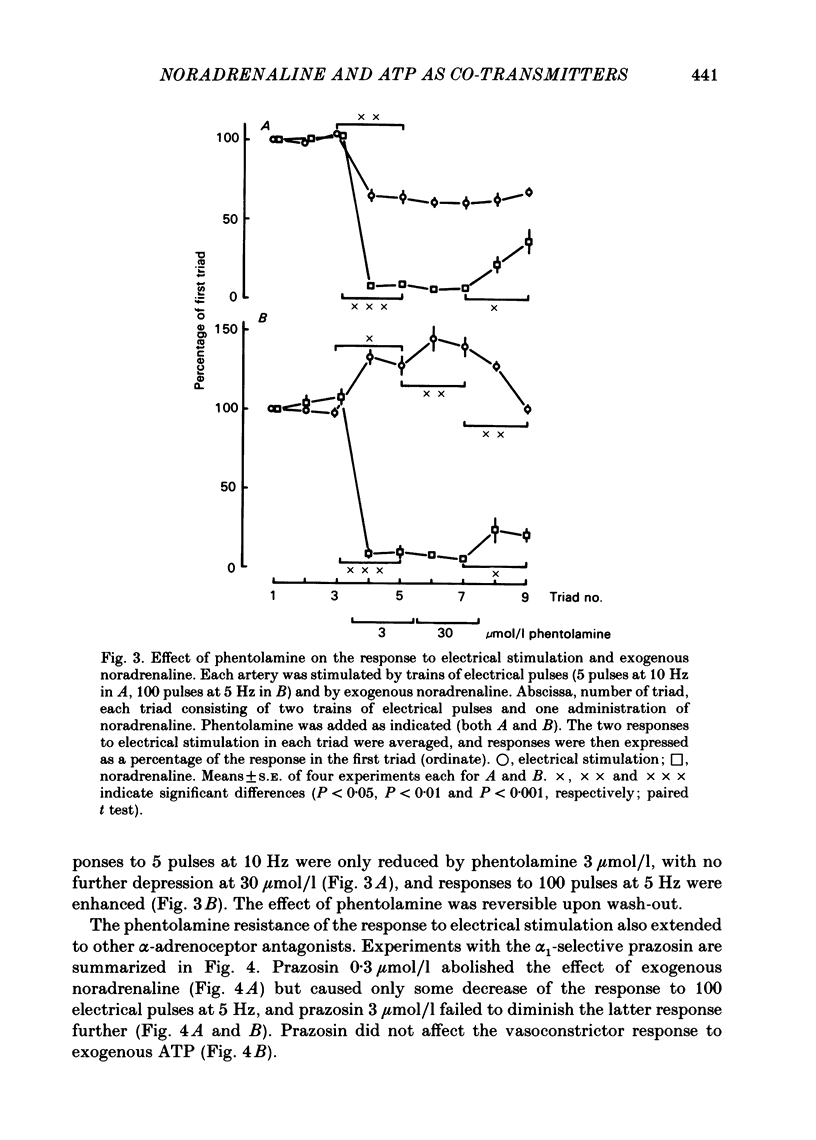
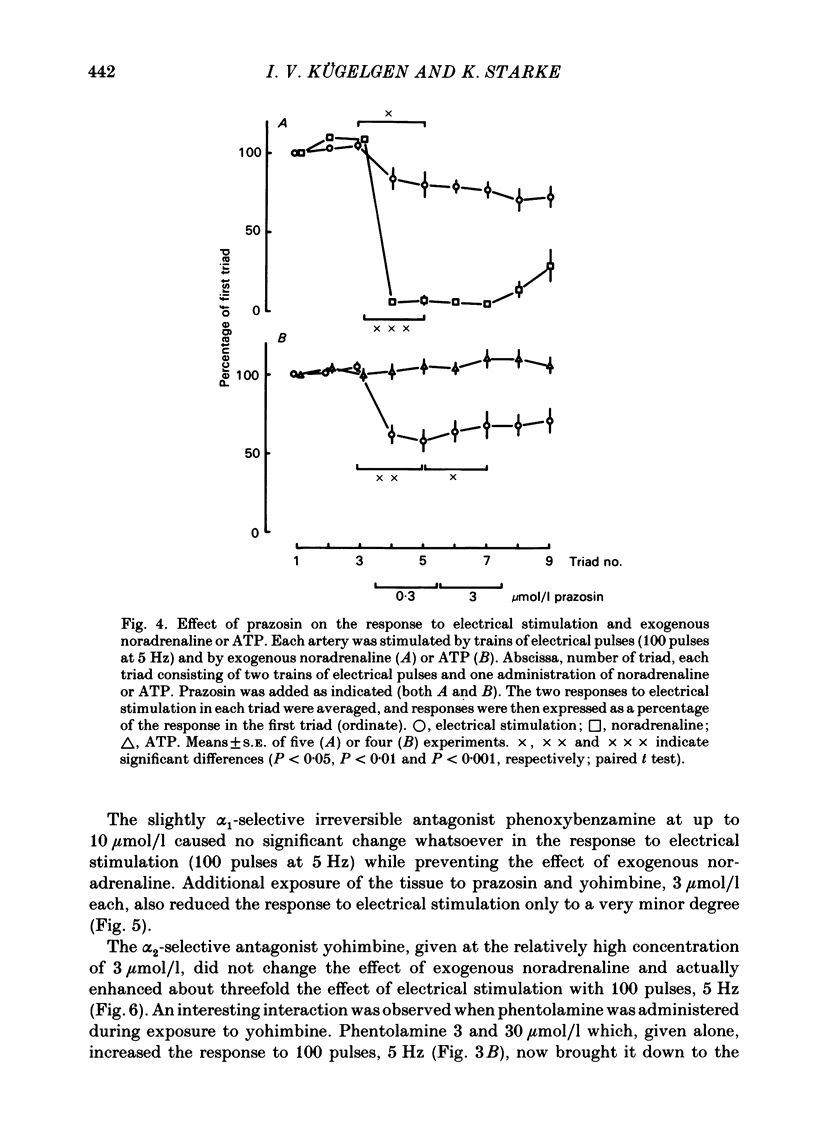
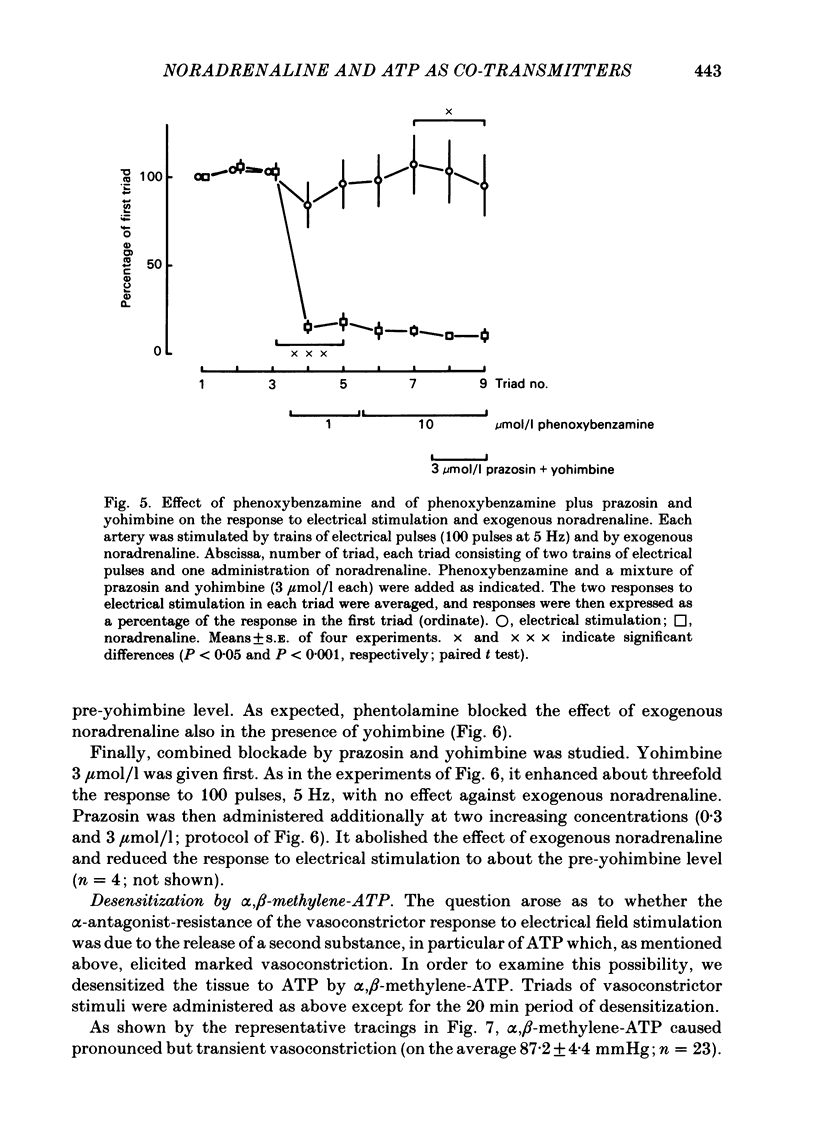
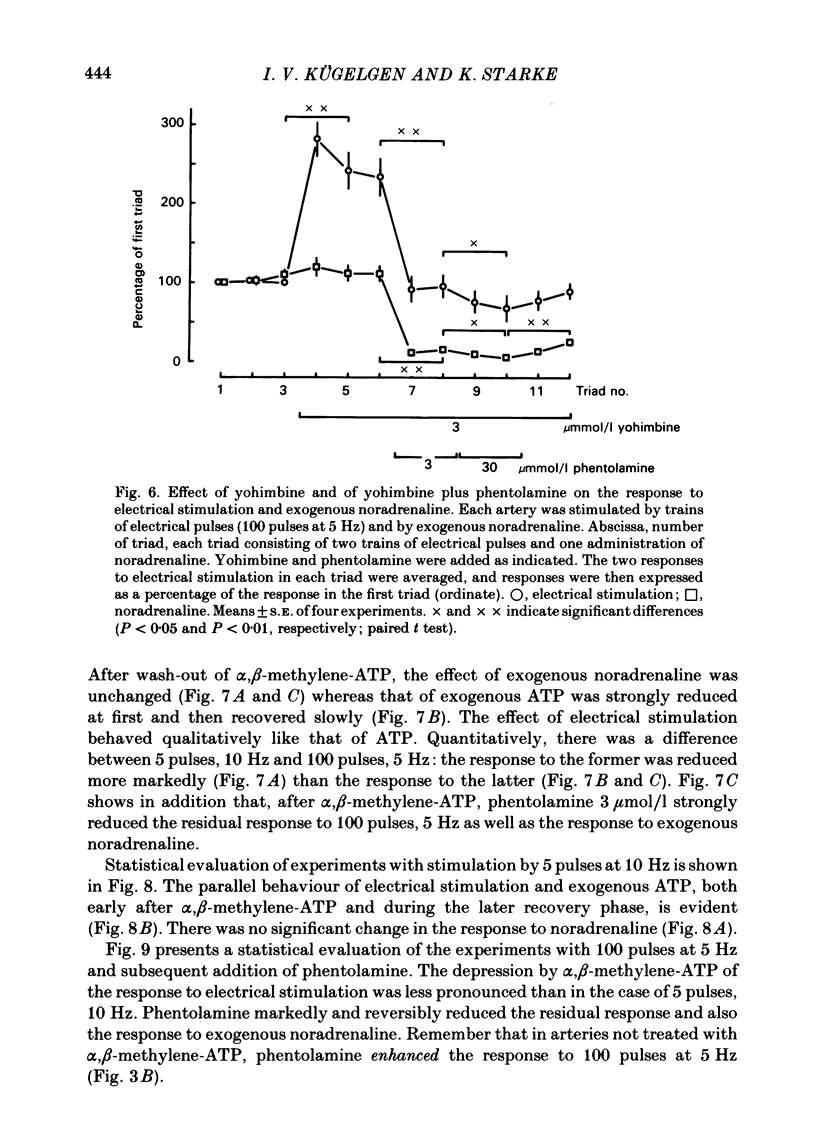
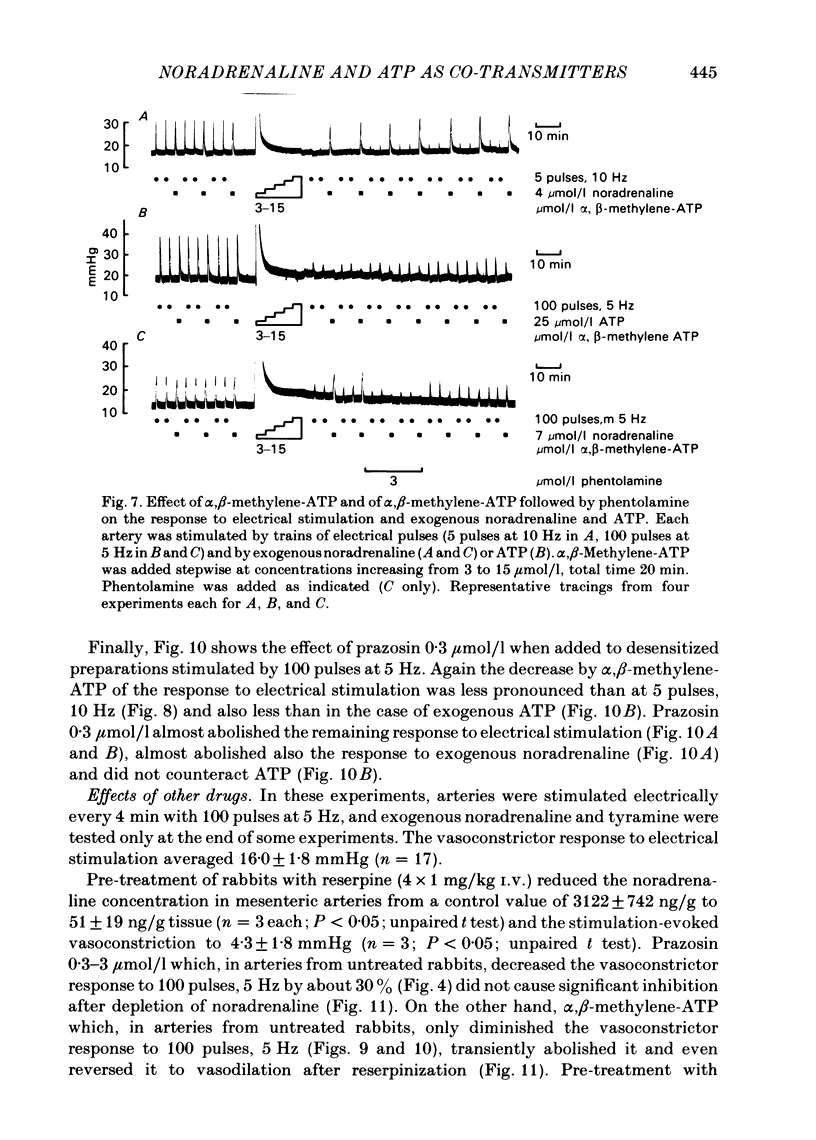
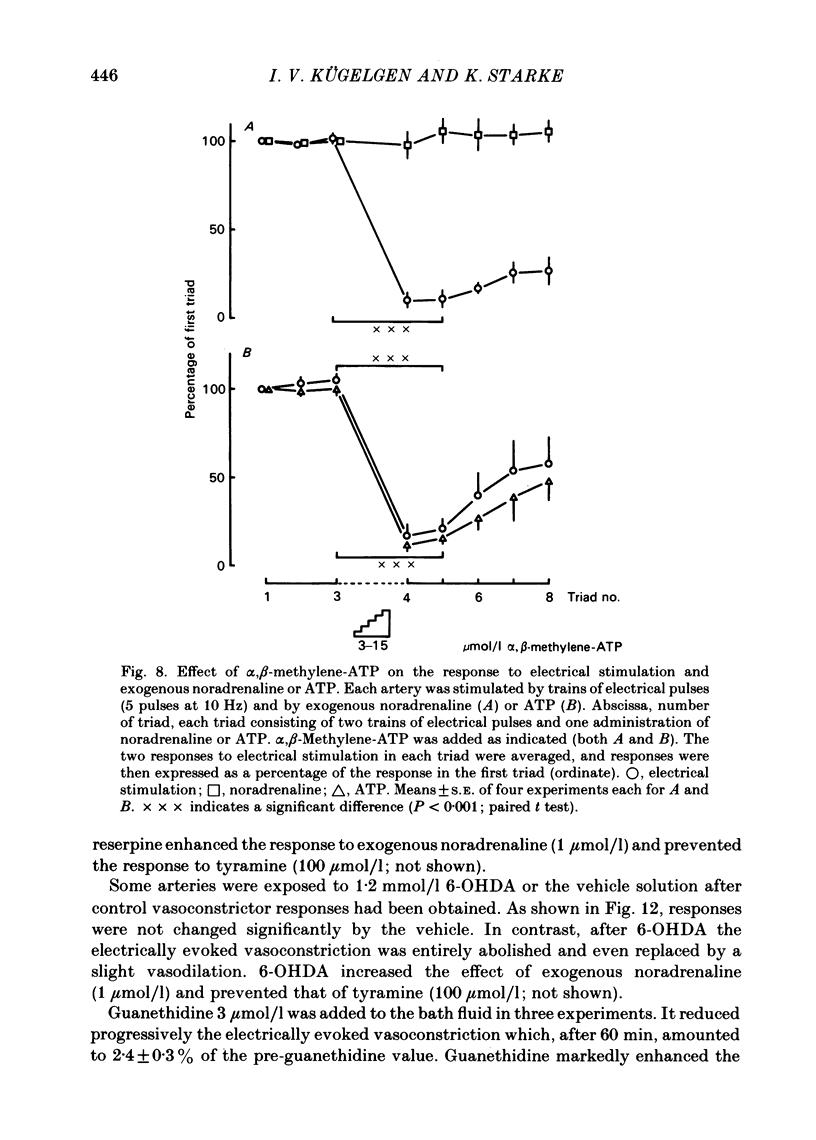
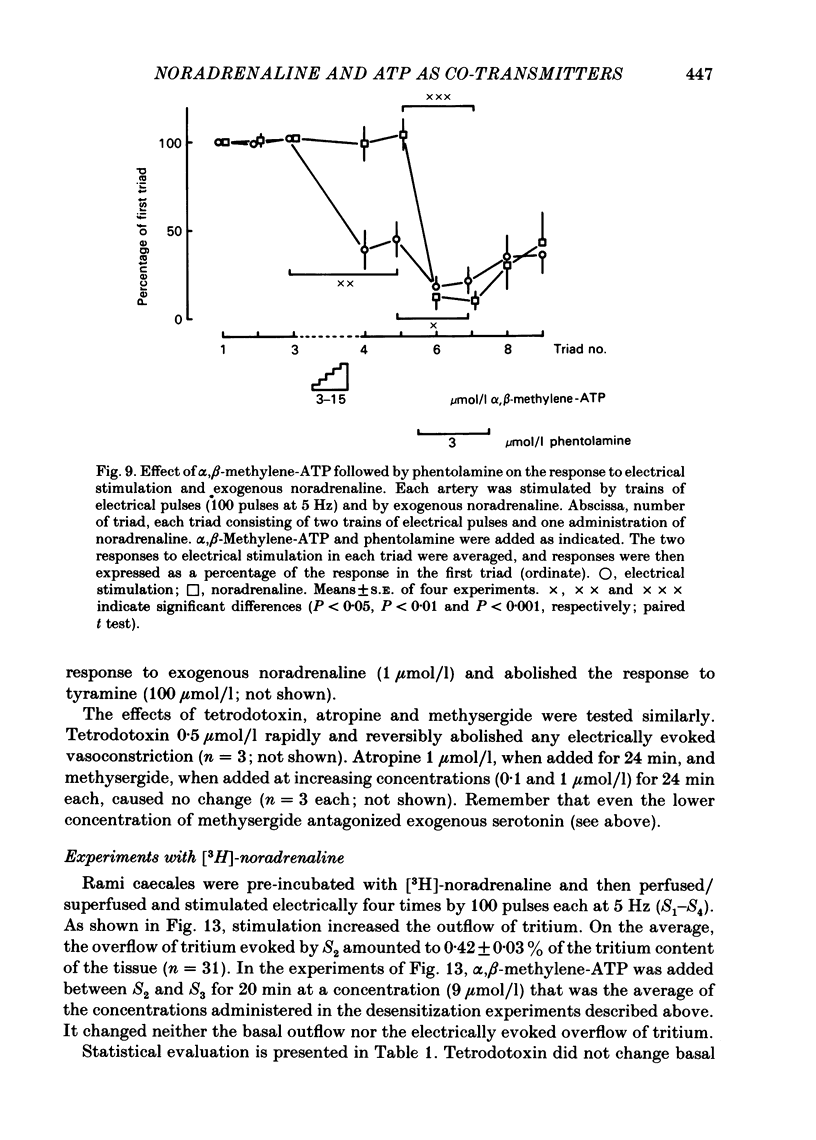
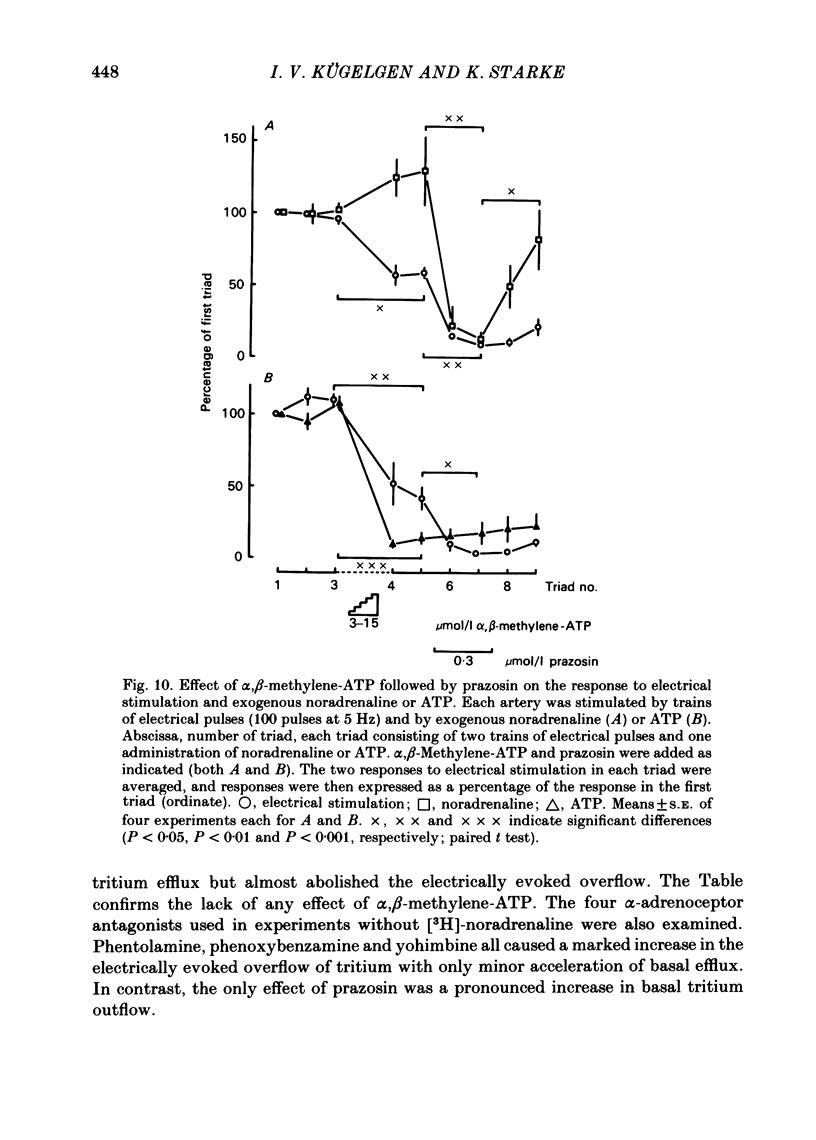
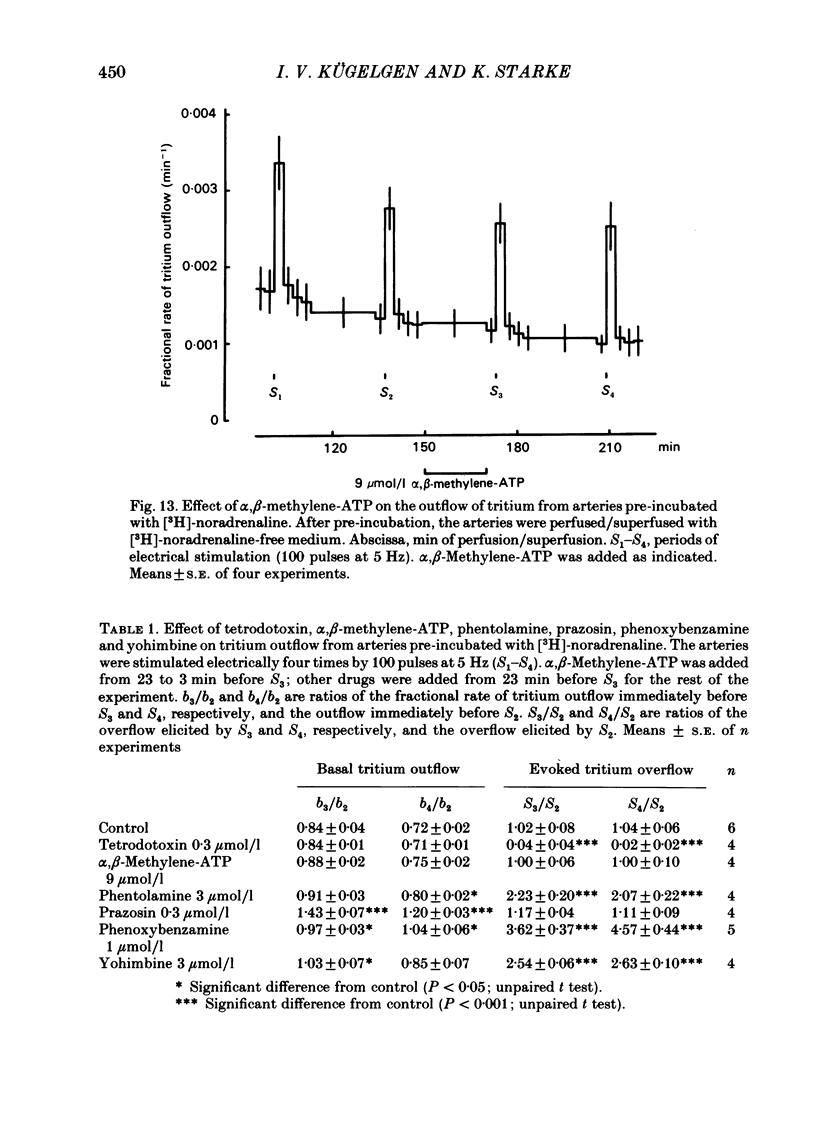
The largest rami caecales of the ileocolic artery, which is a branch of the mesenteric artery, were perfused at a constant rate of flow. Either vasoconstriction (as an increase in perfusion pressure) or the release of previously incorporated [3H]-noradrenaline was measured. Noradrenaline and ATP, but not carbachol, serotonin, adenosine, Arg-vasopressin and neuropeptide Y, caused marked vasoconstriction. When the sympathetic vasoconstrictor axons in the arterial wall were stimulated by electrical field pulses (either 5 pulses at 10 Hz or 100 pulses at 5 Hz; 0.3 ms pulse width, 200 mA current strength), the ensuing vasoconstriction was at best slightly reduced by phentolamine, prazosin and phenoxybenzamine. The response to 100 pulses, 5 Hz was even enhanced by phentolamine and yohimbine. All antagonists except yohimbine blocked the effect of exogenous noradrenaline. Prazosin did not change the effect of exogenous ATP. alpha,beta-Methylene-ATP (3-15 mumol/l) elicited transient vasoconstriction. Subsequently, responses to ATP as well as to electrical stimulation were reduced and recovered slowly. The response to noradrenaline was not changed. That part of the electrically induced vasoconstriction that remained after alpha,beta-methylene-ATP was almost abolished by phentolamine or prazosin. Pre-treatment of the animals with reserpine decreased but did not prevent the electrically evoked contraction of their arteries. The reserpine-resistant response was not changed by prazosin but was abolished by alpha,beta-methylene-ATP. The vasoconstriction elicited by electrical pulses was not affected by atropine or methysergide but was entirely blocked by tetrodotoxin, guanethidine or exposure to 6-hydroxydopamine. In arteries pre-incubated with [3H]-noradrenaline, electrical stimulation (100 pulses at 5 Hz) increased the outflow of tritium. The evoked overflow was blocked by tetrodotoxin, not changed by alpha,beta-methylene-ATP (9 mumol/l) or prazosin, and enhanced by phentolamine, phenoxybenzamine and yohimbine. We conclude that, in the branch of the mesenteric artery examined, both noradrenaline and ATP or a closely related compound transmit information from sympathetic neurones to smooth muscle. An alpha-adrenoceptor antagonist can reduce neurogenic vasoconstriction by blockade of post-junctional alpha-(probably alpha 1) receptors, reserpine by selective depletion of noradrenaline, and alpha,beta-methylene-ATP by desensitization of the post-junctional ATP (probably P2) receptor mechanism. Noradrenaline and ATP appear to be released from the same neurone. In addition, prejunctional alpha 2-adrenergic autoinhibition of transmitter release operates in the artery. alp
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprigliano O., Hermsmeyer K. In vitro denervation of the portal vein and caudal artery of the rat. J Pharmacol Exp Ther. 1976 Sep;198(3):568–577. [PubMed] [Google Scholar]
- Auch-Schwelk W., Starke K., Steppeler A. Experimental conditions required for the enhancement by alpha-adrenoceptor antagonists of noradrenaline release in the rabbit ear artery. Br J Pharmacol. 1983 Mar;78(3):543–551. doi: 10.1111/j.1476-5381.1983.tb08814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Su C. Distribution theory of resistance of neurogenic vasoconstriction to alpha-receptor blockade in the rabbit. Circ Res. 1971 Feb;28(2):179–187. doi: 10.1161/01.res.28.2.179. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976 Aug;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Commarato M. A., Langley A. E., Dugan D. H., Lattime E. C., Smith R. D., Tessman D. K., Kaplan H. R. Prazosin and phentolamine: comparative cardiovascular and autonomic profiles. Clin Exp Hypertens. 1978;1(2):191–217. doi: 10.3109/10641967809068604. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P., Colby J., Westfall D. P. Contribution by purines to the neurogenic response of the vas deferens of the guinea pig. Eur J Pharmacol. 1981 Jan 5;69(1):41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Benton H. P., Lightman S. L., Todd K., Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. A vasopressin-like peptide in the mammalian sympathetic nervous system. Nature. 1984 May 17;309(5965):258–261. doi: 10.1038/309258a0. [DOI] [PubMed] [Google Scholar]
- Hedlund H., Fändriks L., Delbro D., Fasth S. Blockade of non-cholinergic non-adrenergic colonic contraction in response to pelvic nerve stimulation by large doses of alpha, beta-methylene ATP. Acta Physiol Scand. 1983 Dec;119(4):451–454. doi: 10.1111/j.1748-1716.1983.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Ohhashi T., Azuma T. The mode of ATP-induced vasoconstriction in the canine internal carotid artery. Blood Vessels. 1984;21(2):98–104. [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. The presynaptic regulation of noradrenaline release differs in mesenteric arteries of the rabbit and guinea-pig. J Physiol. 1984 Jun;351:379–396. doi: 10.1113/jphysiol.1984.sp015251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger N., Starke K. Further study of prerequisites for the enhancement by alpha-adrenoceptor antagonists of the release of noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):240–246. doi: 10.1007/BF00495950. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Tatemoto K. Pancreatic polypeptide family (APP, BPP, NPY and PYY) in relation to sympathetic vasoconstriction resistant to alpha-adrenoceptor blockade. Acta Physiol Scand. 1982 Dec;116(4):393–402. doi: 10.1111/j.1748-1716.1982.tb07157.x. [DOI] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Mishima S., Miyahara H., Suzuki H. Transmitter release modulated by alpha-adrenoceptor antagonists in the rabbit mesenteric artery: a comparison between noradrenaline outflow and electrical activity. Br J Pharmacol. 1984 Oct;83(2):537–547. doi: 10.1111/j.1476-5381.1984.tb16518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu I., Fujiwara M., Miura A., Sakakibara Y. Possible involvement of adenine nucleotides in sympathetic neuroeffector mechanisms of dog basilar artery. J Pharmacol Exp Ther. 1981 Feb;216(2):401–409. [PubMed] [Google Scholar]
- Sneddon P., Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984 Apr 13;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Alpha-adrenoceptor subclassification. Rev Physiol Biochem Pharmacol. 1981;88:199–236. [PubMed] [Google Scholar]
- Starke K., Hedler L., Steppeler A. Metabolism of endogenous and exogenous noradrenaline in guinea-pig atria. Naunyn Schmiedebergs Arch Pharmacol. 1981 Nov;317(3):193–198. doi: 10.1007/BF00503815. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Discrete events measure single quanta of adenosine 5'-triphosphate secreted from sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1984 Sep;13(1):21–28. doi: 10.1016/0306-4522(84)90256-2. [DOI] [PubMed] [Google Scholar]
- Story D. F., McCulloch M. W., Rand M. J., Standford-Starr C. A. Conditions required for the inhibitory feedback loop in noradrenergic transmission. Nature. 1981 Sep 3;293(5827):62–65. doi: 10.1038/293062a0. [DOI] [PubMed] [Google Scholar]
- Su C. Neurogenic release of purine compounds in blood vessels. J Pharmacol Exp Ther. 1975 Oct;195(1):159–166. [PubMed] [Google Scholar]
- Suzuki H. Electrical responses of smooth muscle cells of the rabbit ear artery to adenosine triphosphate. J Physiol. 1985 Feb;359:401–415. doi: 10.1113/jphysiol.1985.sp015592. [DOI] [PMC free article] [PubMed] [Google Scholar]


