Abstract
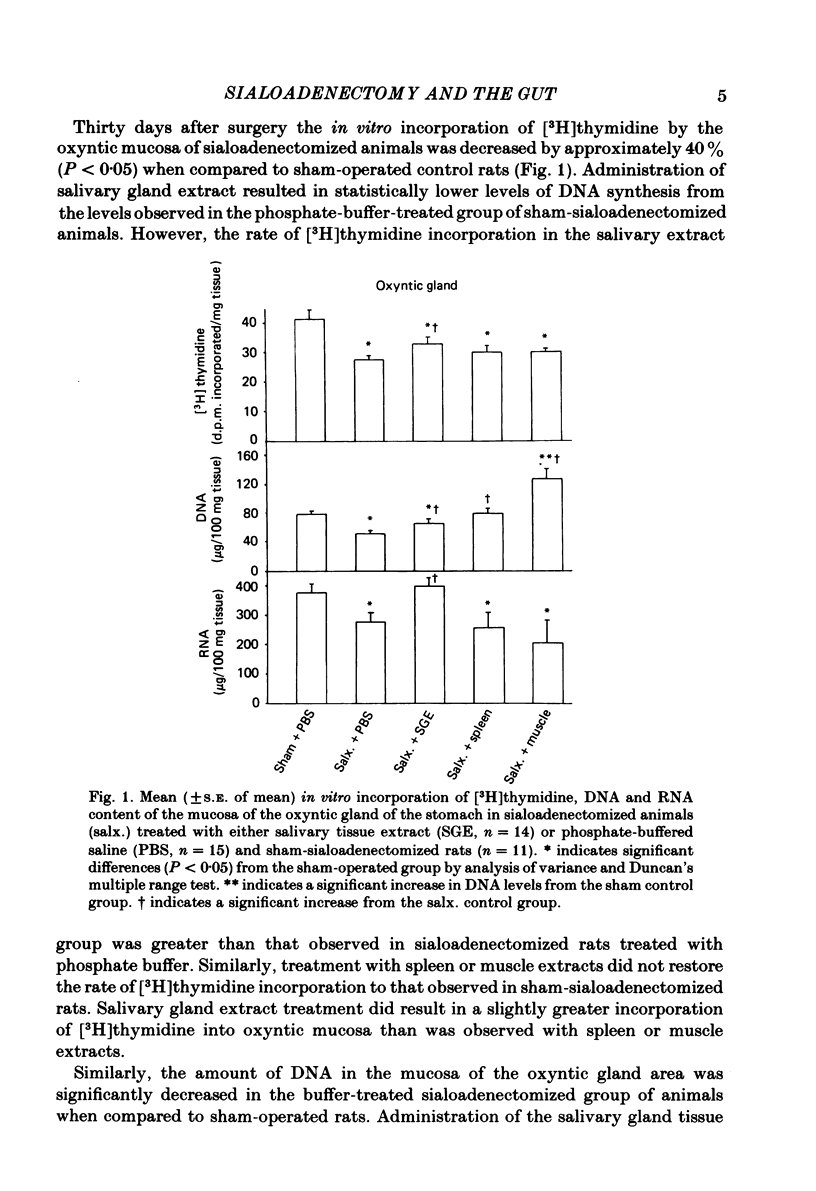
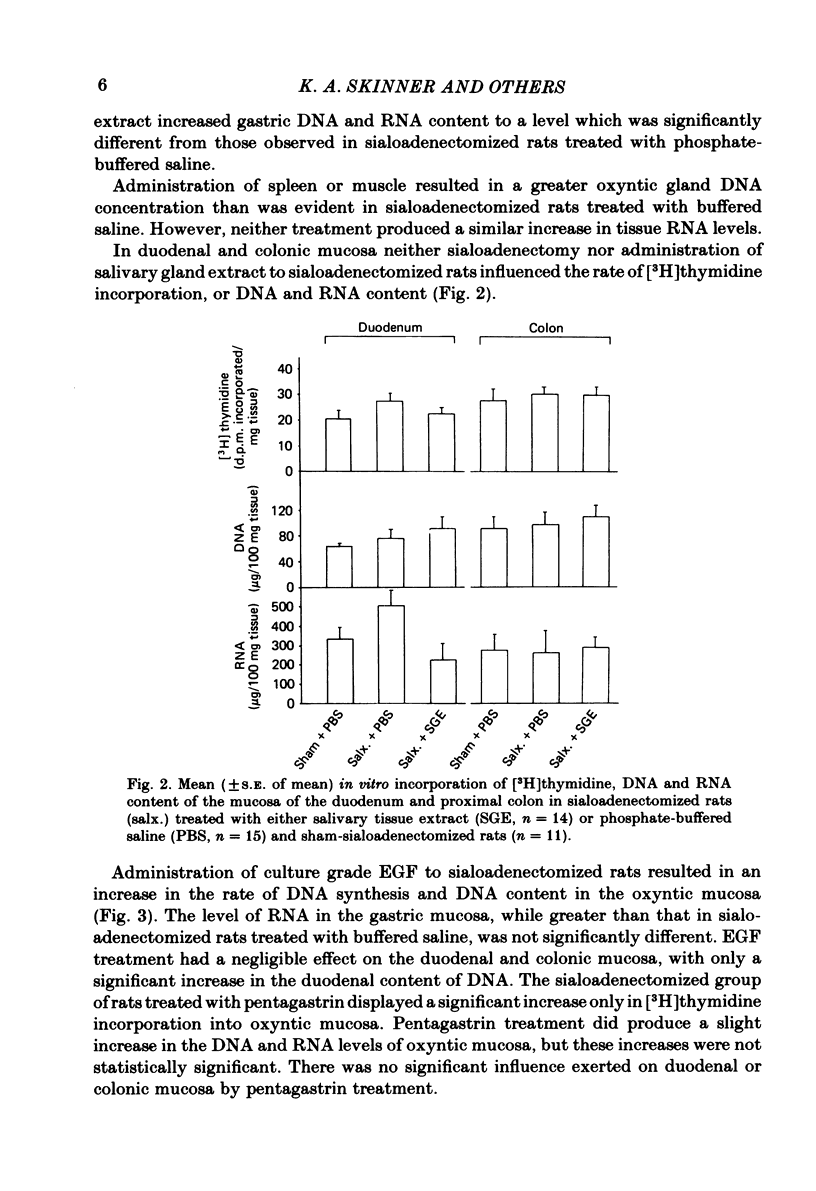
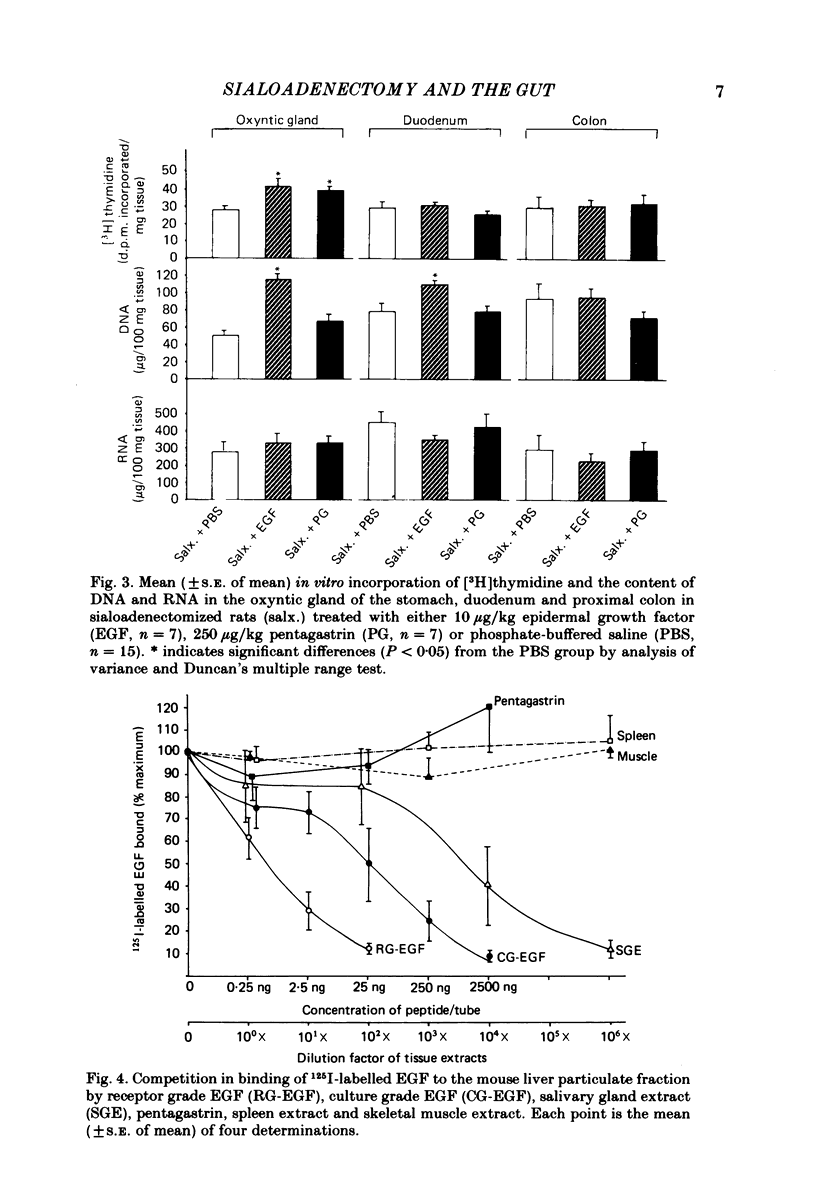
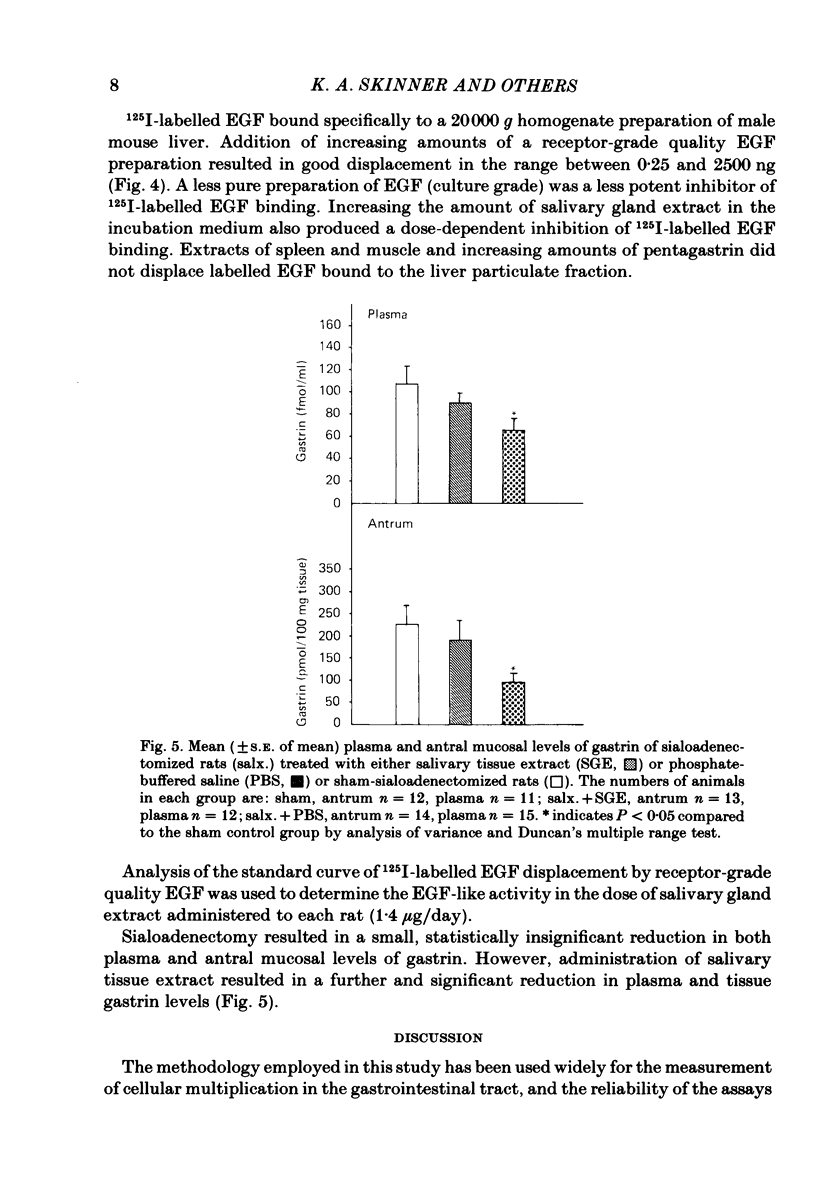
We have examined gastrointestinal mucosal growth 30 days after surgical removal of the submandibular-sublingual salivary glands and ligation of the parotid ducts of rats. The rate of [3H]thymidine uptake in vitro as an estimation of DNA synthesis and the content of DNA and RNA were examined in the oxyntic, duodenal and proximal colonic mucosa. DNA synthesis, DNA and RNA content of oxyntic mucosa were reduced in sialoadenectomized rats when compared to sham-sialoadenectomized control animals. There was no change in the degree of [3H]thymidine incorporation or DNA content of the duodenal or colonic mucosa. Intraperitoneal injection of an aqueous extract of the submandibular-sublingual salivary glands of rats (4.0 mg tissue protein in 0.1 M-sodium phosphate buffer administered twice a day for 15 days) increased the rate of DNA synthesis and the total mucosal DNA and RNA content in the oxyntic mucosa. Injections of extracts of spleen or muscle did not produce consistent results. Administration of epidermal growth factor (10 micrograms/kg) or pentagastrin (250 micrograms/kg) resulted in an increase of the level of DNA synthesis observed in the oxyntic mucosa of sialoadenectomized rats. Plasma and antral tissue levels of the trophic hormone, gastrin, were not significantly decreased in sialoadenectomized rats treated with 0.1 M-sodium phosphate-buffered saline. However, treatment with the salivary tissue extract did result in significant reductions in both plasma and tissue levels of gastrin. We conclude that elimination of the major salivary glands in the rat results in a decrease in [3H]thymidine uptake and DNA content of the gastric oxyntic mucosa. These effects are not mediated via a reduction in endogenous levels of the trophic hormone, gastrin. Administration of an aqueous extract of salivary tissue exerted a small but significant trophic influence on the oxyntic mucosa of the rat.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMANO M., MESSIER B., LEBLOND C. P. Specificity of labelled thymidine as a deoxyribonucleic acid precursor in radioautography. J Histochem Cytochem. 1959 May;7(3):153–155. doi: 10.1177/7.3.153. [DOI] [PubMed] [Google Scholar]
- BUEKER E. D., SCHENKEIN I. EFFECTS OF DAILY SUBCUTANEOUS INJECTIONS OF NERVE GROWTH STIMULATING PROTEIN FRACTIONS ON MICE DURING POSTNATAL TO ADULT STAGE. Ann N Y Acad Sci. 1964 Oct 9;118:183–205. doi: 10.1111/j.1749-6632.1964.tb33980.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- COHEN S., ELLIOTT G. A. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J Invest Dermatol. 1963 Jan;40:1–5. doi: 10.1038/jid.1963.1. [DOI] [PubMed] [Google Scholar]
- EPSTEIN A. N., SPECTOR D., SAMMAN A., GOLDBLUM C. EXAGGERATED PRANDIAL DRINKING IN THE RAT WITHOUT SALIVARY GLANDS. Nature. 1964 Mar 28;201:1342–1343. doi: 10.1038/2011342a0. [DOI] [PubMed] [Google Scholar]
- Feldman E. J., Aures D., Grossman M. I. Epidermal growth factor stimulates ornithine decarboxylase activity in the digestive tract of mouse. Proc Soc Exp Biol Med. 1978 Dec;159(3):400–402. doi: 10.3181/00379727-159-40357. [DOI] [PubMed] [Google Scholar]
- Johnson L. R., Guthrie P. D. Mucosal DNA synthesis: a short term index of the trophic action of gastrin. Gastroenterology. 1974 Sep;67(3):453–459. [PubMed] [Google Scholar]
- Johnson L. R. The trophic action of gastrointestinal hormones. Gastroenterology. 1976 Feb;70(2):278–288. [PubMed] [Google Scholar]
- Kissileff H. R. Food-associated drinking in the rat. J Comp Physiol Psychol. 1969 Mar;67(3):284–300. doi: 10.1037/h0026773. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Brzozowski T., Piastucki I., Dembiński A., Dembińska-Kieć A., Zmuda A., Gryglewski R., Gregory H. Gastric cytoprotection by epidermal growth factor. Role of endogenous prostaglandins and DNA synthesis. Gastroenterology. 1981 Sep;81(3):438–443. [PubMed] [Google Scholar]
- Li A. K., Schattenkerk M. E., Huffman R. G., Ross J. S., Malt R. A. Hypersecretion of submandibular saliva in male mice: trophic response in small intestine. Gastroenterology. 1983 May;84(5 Pt 1):949–955. [PubMed] [Google Scholar]
- Lichtenberger L., Miller L. R., Erwin D. N., Johnson L. R. Effect of pentagastrin on adult rat duodenal cells in culture. Gastroenterology. 1973 Aug;65(2):242–251. [PubMed] [Google Scholar]
- Lichtenberger L., Welsh J. D., Johnson L. R. Relationship between the changes in gastrin levels and intestinal properties in the starved rat. Am J Dig Dis. 1976 Jan;21(1):33–38. doi: 10.1007/BF01074136. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Malo C., Ménard D. Influence of epidermal growth factor on the development of suckling mouse intestinal mucosa. Gastroenterology. 1982 Jul;83(1 Pt 1):28–35. [PubMed] [Google Scholar]
- Martinez-Hernandezo A., Nakane P. K., Pierce G. B. Relationships between the submaxillary gland and the thymus. Lab Invest. 1973 Aug;29(2):266–271. [PubMed] [Google Scholar]
- Maurer H. R. Potential pitfalls of [3H]thymidine techniques to measure cell proliferation. Cell Tissue Kinet. 1981 Mar;14(2):111–120. doi: 10.1111/j.1365-2184.1981.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Rubin B. Production and evaluation of antibodies for the radioimmunoassay of gastrin. Scand J Clin Lab Invest. 1972 Oct;30(2):221–232. doi: 10.3109/00365517209081114. [DOI] [PubMed] [Google Scholar]
- Skinner K. A., Tepperman B. L. Influence of desalivation on acid secretory output and gastric mucosal integrity in the rat. Gastroenterology. 1981 Aug;81(2):335–339. [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Determination of gastrin in serum. An evaluation of the reliability of a radioimmunoassay. Scand J Gastroenterol. 1973;8(2):101–112. [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Preparation of 125 I-labelled synthetic human gastrin I for radioimmunoanalysis. Scand J Clin Lab Invest. 1972 Dec;30(4):361–368. doi: 10.3109/00365517209080271. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Johnson L. R. Pentagastrin protects against stress ulceration in rats. Gastroenterology. 1979 Feb;76(2):327–334. [PubMed] [Google Scholar]
- Velasco Plaza A., Menendez-Patterson A., Marin B. Effects of the extirpation of submandibular salivary glands on the oxidative activity of nervous and glandular structures in the male rat. Arch Oral Biol. 1979;24(4):245–247. doi: 10.1016/0003-9969(79)90084-0. [DOI] [PubMed] [Google Scholar]


