Abstract
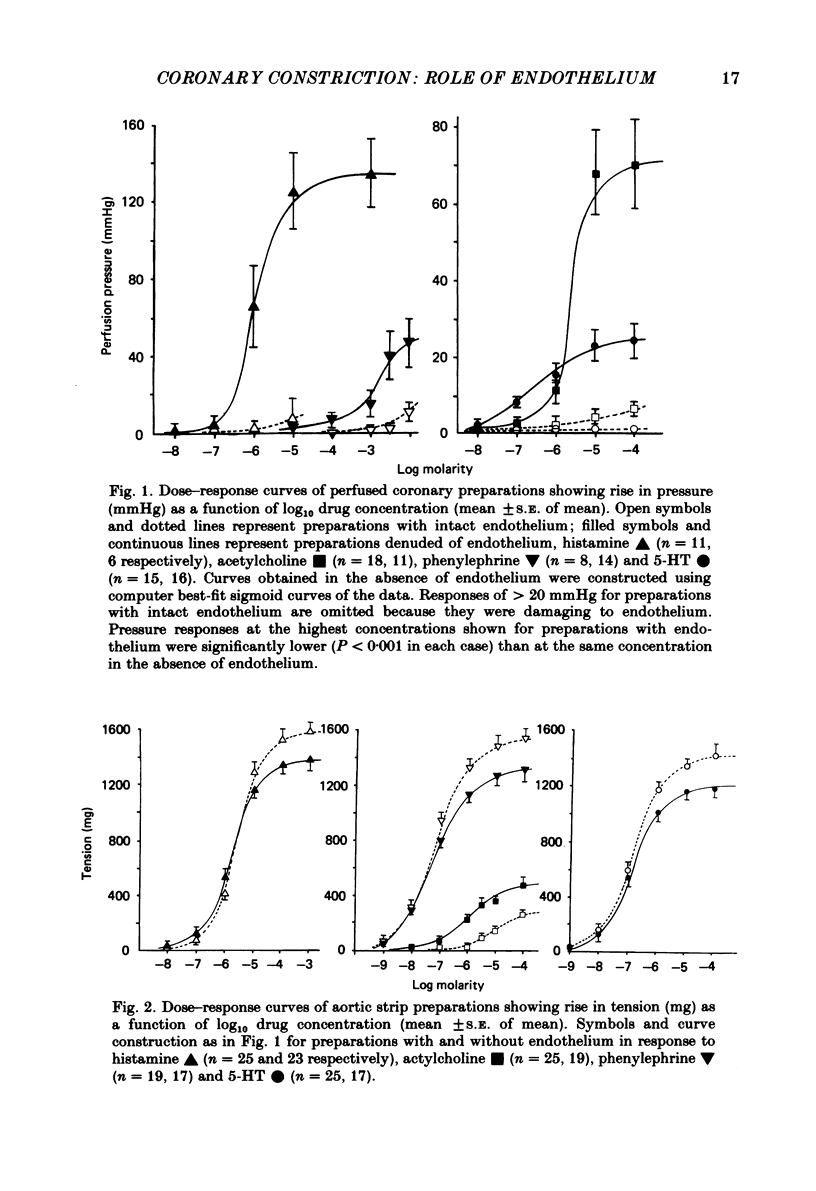
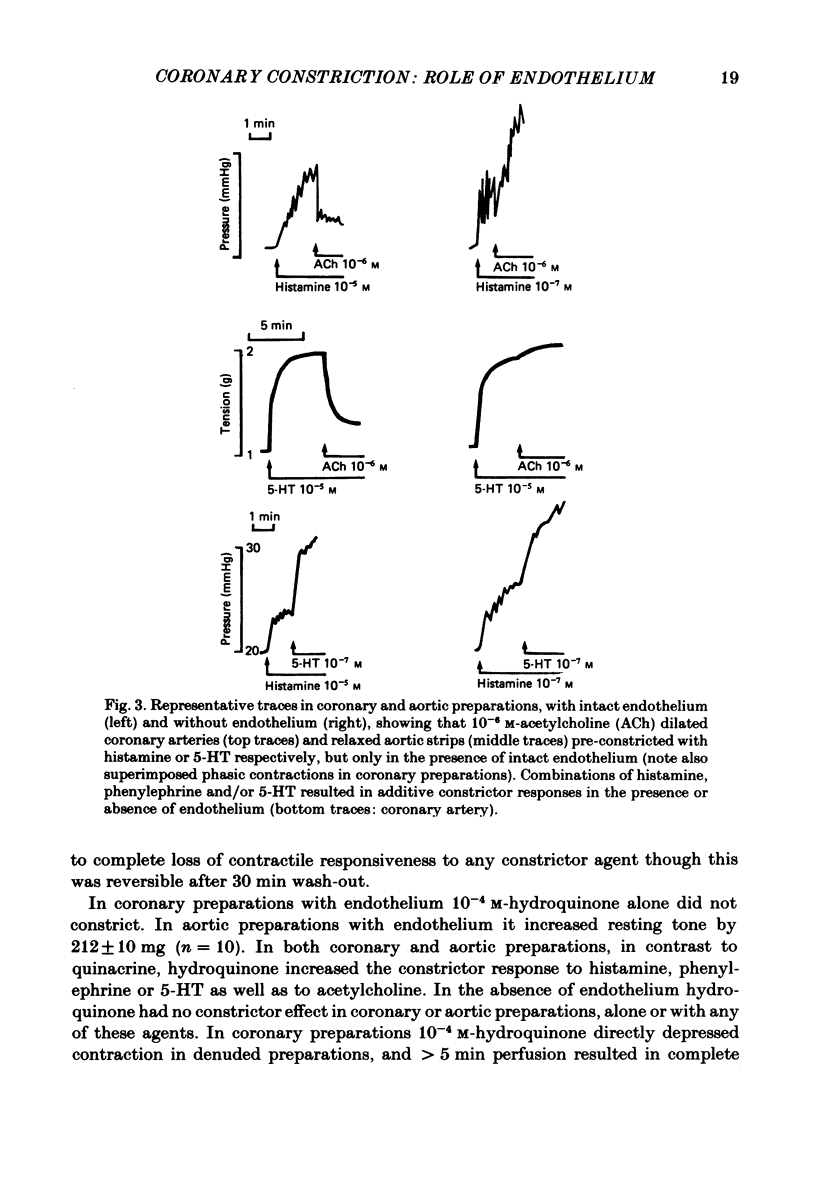
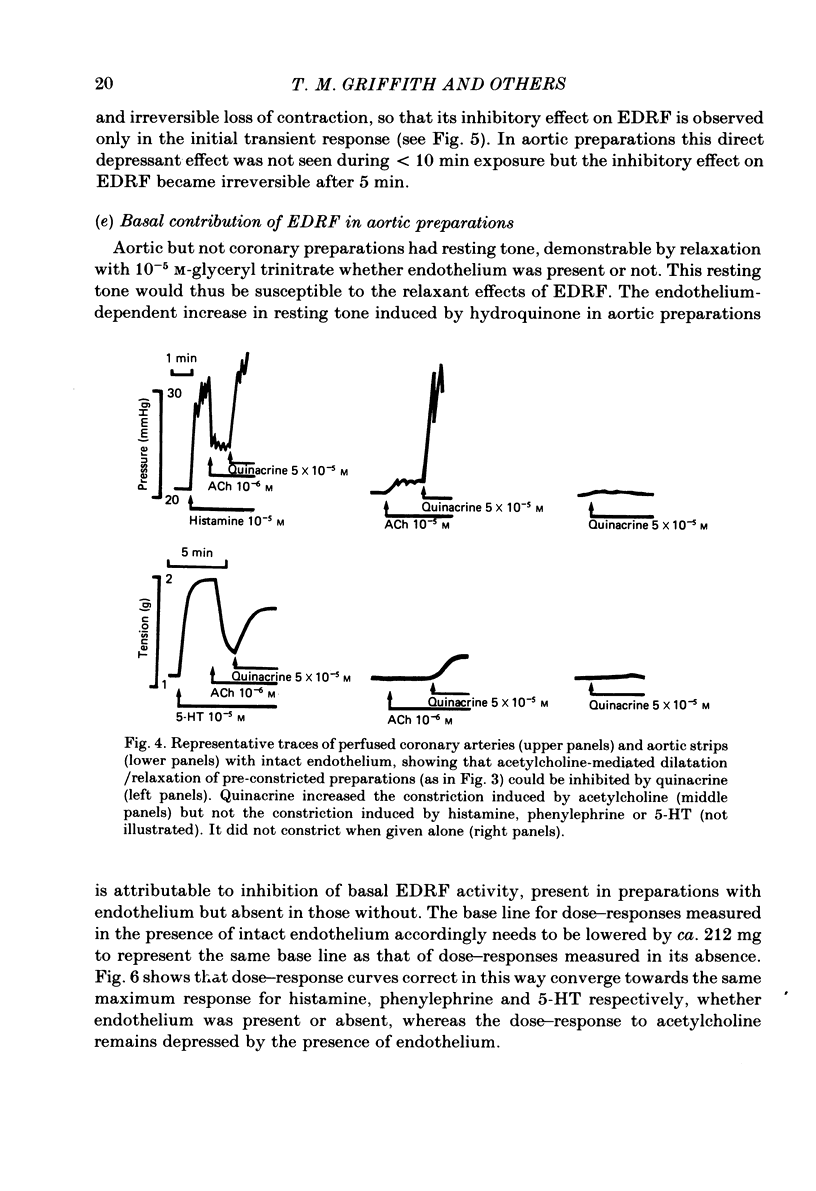
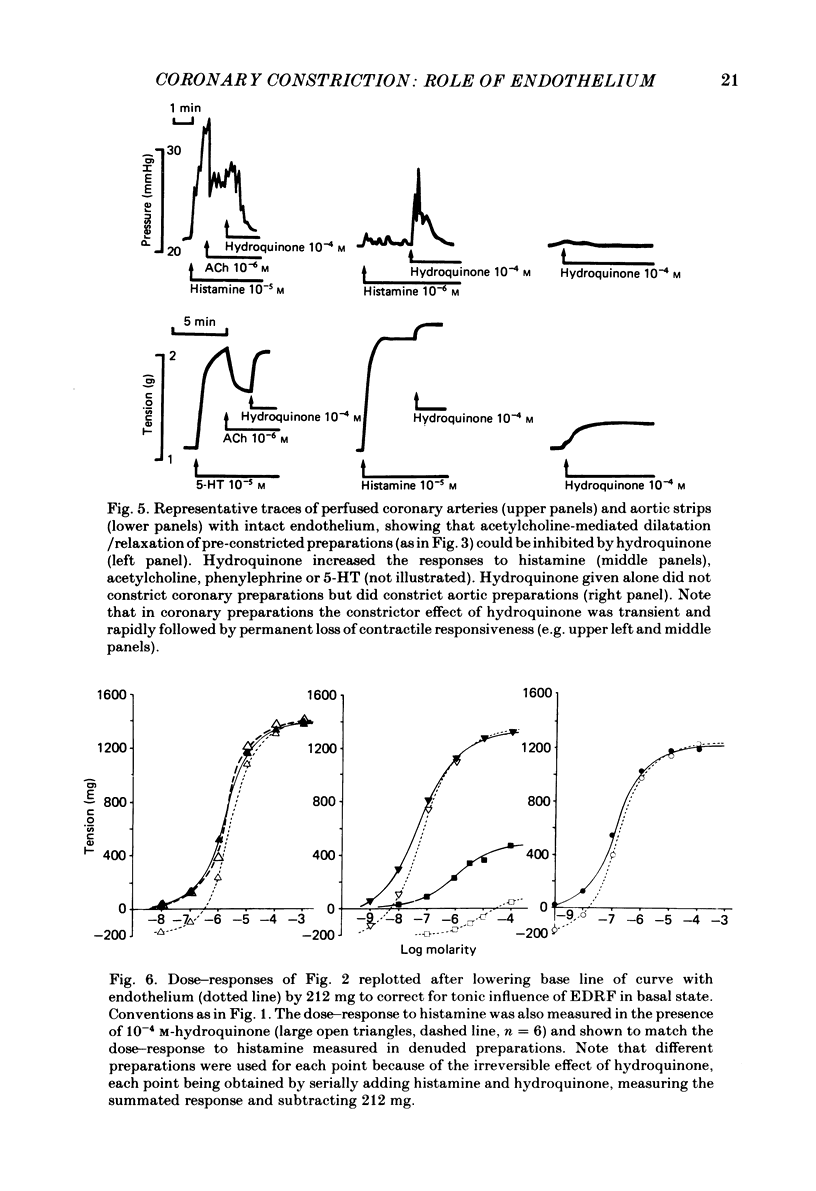
Isolated perfused coronary arteries and aortic ring preparations of rabbits were studied, both with intact endothelium and with endothelium removed by K-rich solution and friction respectively. Constrictor dose-responses to histamine, acetylcholine, phenylephrine and 5-hydroxytryptamine (5-HT) were measured. They were greatly depressed by the presence of endothelium in coronary preparations. In aortic preparations endothelium affected dose-responses relatively little, depressing the response to acetylcholine but apparently increasing the responses to the other three agents. Acetylcholine relaxed pre-constricted coronary or aortic preparations but only when endothelium was present. This relaxation was inhibited by quinacrine or hydroquinone. Aortic preparations had resting tone which could be increased by hydroquinone if endothelium was present, suggesting continual release of endothelium-derived relaxant factor (EDRF) at rest. When allowance was made for basal EDRF activity in aortic preparations, the maximal constrictor response to acetylcholine remained lower in the presence of endothelium, consistent with acetylcholine stimulation of EDRF, but maximal constrictor responses to the other three agents were the same with and without endothelium, suggesting that the direct constrictor response overrides EDRF activity.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks T. M., Angus J. A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983 Oct 13;305(5935):627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Role of the intima in cholinergic and purinergic relaxation of isolated canine femoral arteries. J Physiol. 1981 Jul;316:347–355. doi: 10.1113/jphysiol.1981.sp013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. W., Slack B. E. Sensitivity to indomethacin of tetrodotoxin-resistant contractions of smooth muscle from the base of rabbit bladder. Br J Pharmacol. 1983 Jun;79(2):334–336. doi: 10.1111/j.1476-5381.1983.tb11005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S. E., Talbot T. L. Dynamic coronary tone in precipitation, exacerbation and relief of angina pectoris. Am J Cardiol. 1981 Oct;48(4):797–803. doi: 10.1016/0002-9149(81)90160-0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Sato M., Ohashi M., Metz M. Z., Bing R. J. Inhibitory effect of a calcium antagonist (diltiazem) on aortic and coronary contractions in rabbits. J Mol Cell Cardiol. 1982 Dec;14(12):741–744. doi: 10.1016/0022-2828(82)90187-0. [DOI] [PubMed] [Google Scholar]
- Van de Voorde J., Leusen I. Vascular endothelium and the relaxation effect of histamine on aorta of the rat. Arch Int Pharmacodyn Ther. 1982 Apr;256(2):329–330. [PubMed] [Google Scholar]
- Wiener L., Kasparian H., Duca P. R., Walinsky P., Gottlieb R. S., Hanckel F., Brest A. N. Spectrum of coronary arterial spasm. Clinical, angiographic and myocardial metabolic experience in 29 cases. Am J Cardiol. 1976 Dec;38(7):945–955. doi: 10.1016/0002-9149(76)90808-0. [DOI] [PubMed] [Google Scholar]



