Abstract
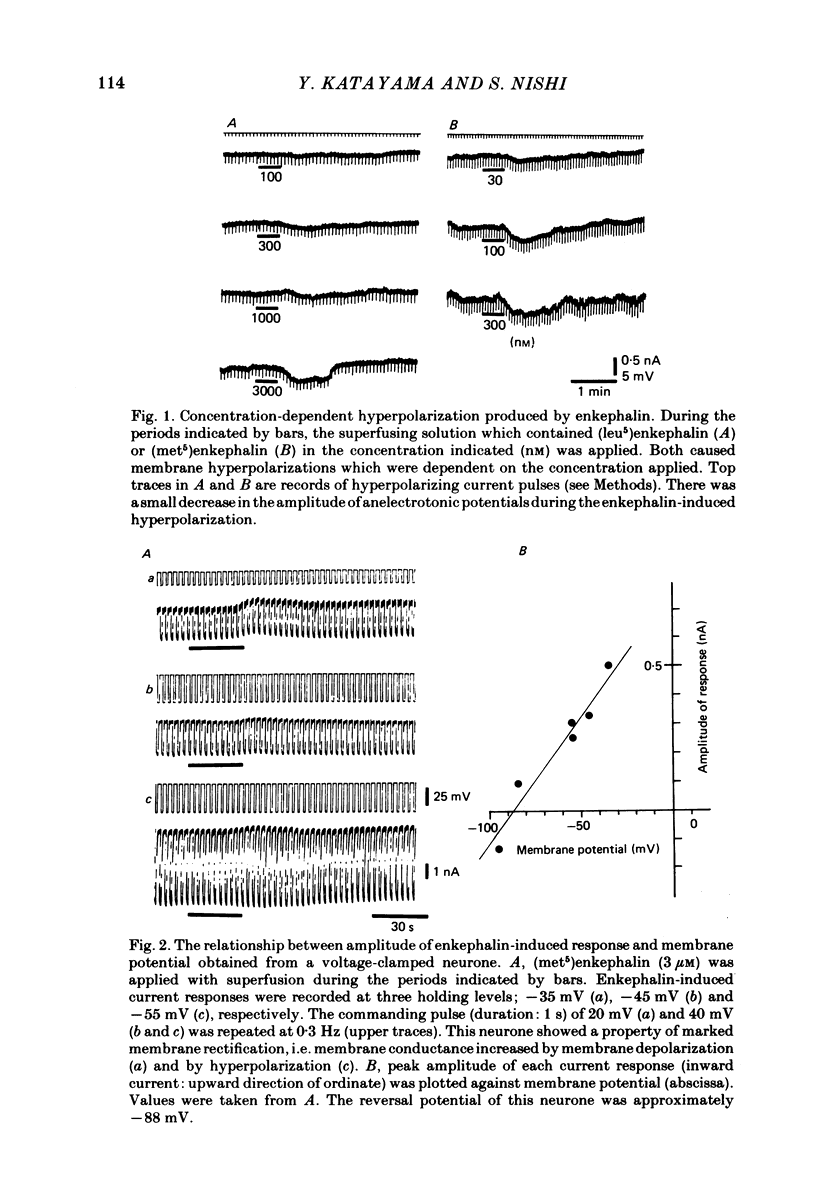
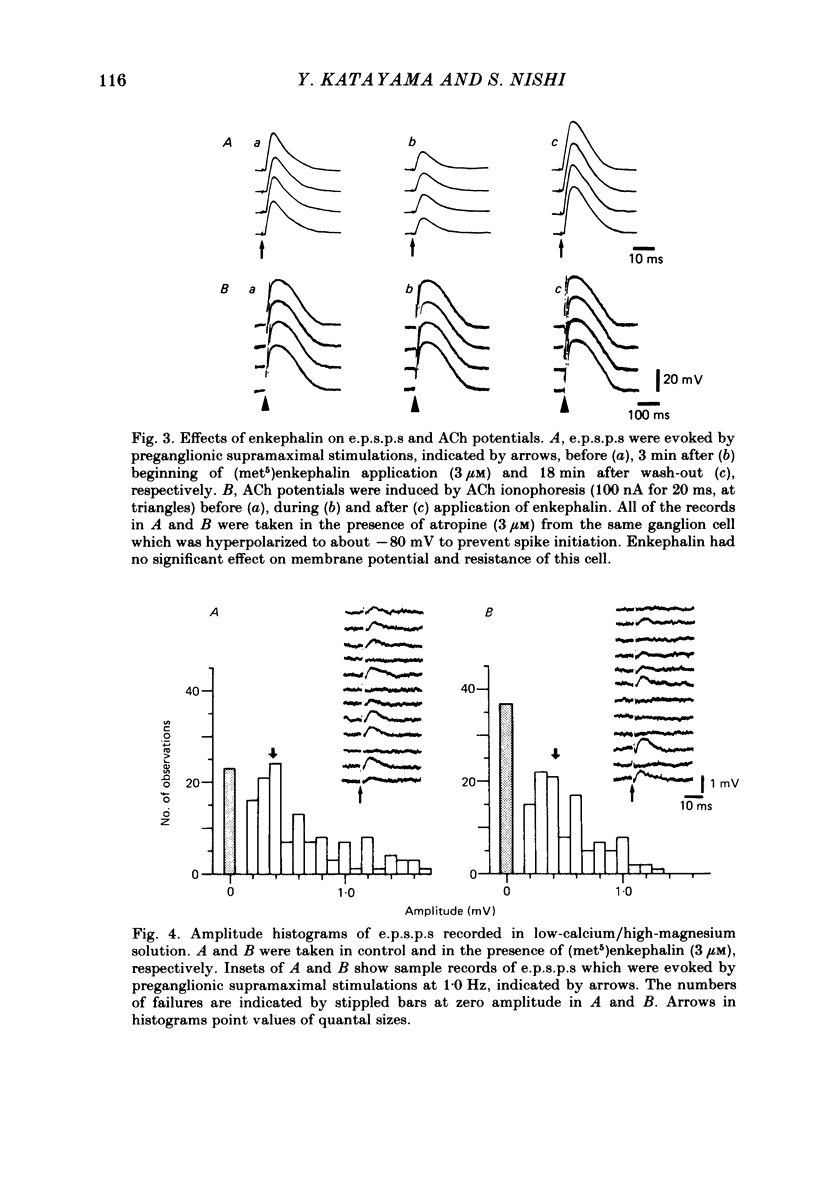
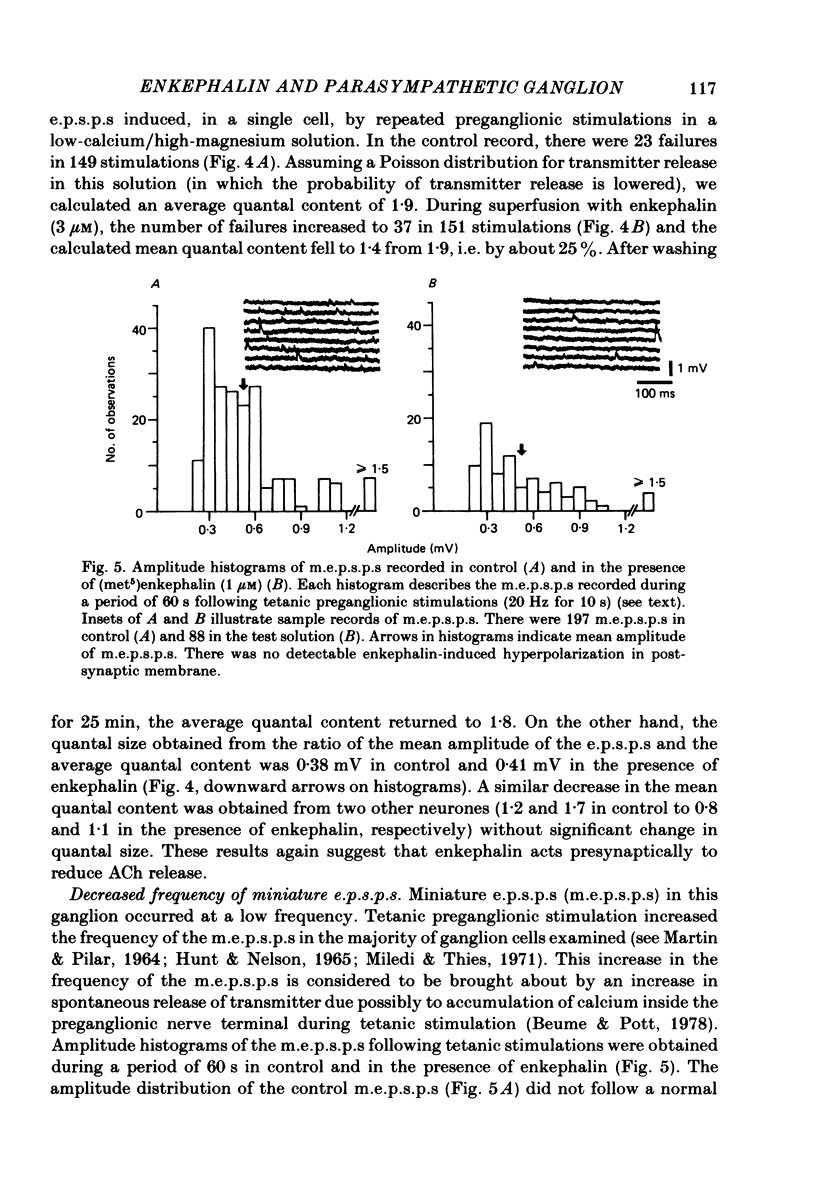
Intracellular recordings were made in vitro from neurones of the cat parasympathetic ciliary ganglion with a current- or voltage-clamp technique. (Met5)enkephalin and (leu5)enkephalin (10 nM to 10 microM) were applied by superfusion. Both caused a membrane hyperpolarization which persisted in a calcium-free/high-magnesium solution, and both reduced the amplitude of excitatory post-synaptic potentials (e.p.s.p.s). These actions of enkephalin were antagonized by naloxone. The enkephalin-induced hyperpolarization was associated with an increase in membrane conductance, reversed in polarity at -90 mV and was not altered by changing external sodium and chloride concentrations. This indicates that the enkephalin hyperpolarization is due mainly to activation of potassium conductance. Enkephalin decreased the mean quantal content of e.p.s.p.s recorded in low-calcium/high-magnesium solution, without changing quantal size. Furthermore, the increase in the frequency of miniature e.p.s.p.s after tetanic preganglionic stimulations was inhibited by enkephalin. Acetylcholine potentials were not altered by enkephalin. These findings suggest that enkephalin reduces transmitter release. The experiments suggest that enkephalin may inhibit ganglionic transmission by both pre- and post-synaptic actions in a mammalian parasympathetic ganglion.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumont A., Hughes J. Biology of opioid peptides. Annu Rev Pharmacol Toxicol. 1979;19:245–267. doi: 10.1146/annurev.pa.19.040179.001333. [DOI] [PubMed] [Google Scholar]
- Beume R., Pott L. Dual effect of external calcium on the frequency of miniature synaptic potentials in frog sympathetic ganglion cells. Pflugers Arch. 1978 Aug 25;376(1):21–26. doi: 10.1007/BF00585243. [DOI] [PubMed] [Google Scholar]
- Bixby J. L., Spitzer N. C. Enkephalin reduces quantal content at the frog neuromuscular junction. Nature. 1983 Feb 3;301(5899):431–432. doi: 10.1038/301431a0. [DOI] [PubMed] [Google Scholar]
- Bornstein J. C., Fields H. L. Morphine presynaptically inhibits a ganglionic cholinergic synapse. Neurosci Lett. 1979 Nov;15(1):77–82. doi: 10.1016/0304-3940(79)91532-5. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Erichsen J. T., Reiner A., Karten H. J. Co-occurrence of substance P-like and Leu-enkephalin-like immunoreactivities in neurones and fibres of avian nervous system. Nature. 1982 Feb 4;295(5848):407–410. doi: 10.1038/295407a0. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Kado R. T. Voltage-current relationship of a carbachol-induced potassium-ion pathway in Aplysia neurones. J Physiol. 1975 Mar;245(3):713–725. doi: 10.1113/jphysiol.1975.sp010870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H., 3rd, Gallagher J. P., Shinnick-Gallagher P. An intracellular investigation of cat vesical pelvic ganglia. J Neurophysiol. 1980 Feb;43(2):343–354. doi: 10.1152/jn.1980.43.2.343. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., NELSON P. G. STRUCTURAL AND FUNCTIONAL CHANGES IN THE FROG SYMPATHETIC GANGLION FOLLOWING CUTTING OF THE PRESYNAPTIC NERVE FIBRES. J Physiol. 1965 Mar;177:1–20. doi: 10.1113/jphysiol.1965.sp007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Ryall R. W. Is excitation by enkephalins of hippocampal neurones in the rat due to presynaptic facilitation or to disinhibition? J Physiol. 1980 Nov;308:315–330. doi: 10.1113/jphysiol.1980.sp013473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Higashi H., Shinnick-Gallagher P., Gallagher J. P. Morphine enhances and depresses Ca2+-dependent responses in visceral primary afferent neurons. Brain Res. 1982 Nov 11;251(1):186–191. doi: 10.1016/0006-8993(82)91291-4. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Purves D. Post-natal reduction of neural unit size in the rabbit ciliary ganglion. J Physiol. 1981 Sep;318:143–159. doi: 10.1113/jphysiol.1981.sp013855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., Nishi S. Voltage-clamp analysis of peptidergic slow depolarizations in bullfrog sympathetic ganglion cells. J Physiol. 1982 Dec;333:305–313. doi: 10.1113/jphysiol.1982.sp014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Yui R. An electron microscopic study on enkephalin-like immunoreactive nerve fibers in the celiac ganglion of guinea pigs. Brain Res. 1982 Dec 2;252(1):142–145. doi: 10.1016/0006-8993(82)90987-8. [DOI] [PubMed] [Google Scholar]
- Konishi S., Tsunoo A., Otsuka M. Enkephalins presynaptically inhibit cholinergic transmission in sympathetic ganglia. Nature. 1979 Nov 29;282(5738):515–516. doi: 10.1038/282515a0. [DOI] [PubMed] [Google Scholar]
- Lee H. K., Wang S. C. Mechanism of morphine-induced miosis in the dog. J Pharmacol Exp Ther. 1975 Feb;192(2):415–431. [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa L. M., Prince D. A. Enkephalin inhibition of inhibitory input to CA1 and CA3 pyramidal neurons in the hippocampus. Brain Res. 1982 Oct 14;249(2):271–280. doi: 10.1016/0006-8993(82)90061-0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A. Opiate activation of potassium conductance in myenteric neurons: inhibition by calcium ion. Brain Res. 1982 Jun 17;242(1):145–150. doi: 10.1016/0006-8993(82)90504-2. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A. Opiates and enkephalin reduce the excitability of neuronal processes. Neuroscience. 1981;6(10):1943–1951. doi: 10.1016/0306-4522(81)90034-8. [DOI] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. B., Tallarida R. J. Pupillographic analysis of morphine action in the rabbit: role to the autonomic nervous system. Eur J Pharmacol. 1982 May 21;80(2-3):197–202. doi: 10.1016/0014-2999(82)90054-1. [DOI] [PubMed] [Google Scholar]
- North R. A., Katayama Y., Williams J. T. On the mechanism and site of action of enkephalin on single myenteric neurons. Brain Res. 1979 Apr 6;165(1):67–77. doi: 10.1016/0006-8993(79)90045-3. [DOI] [PubMed] [Google Scholar]
- North R. A., Tonini M. The mechanism of action of narcotic analgesics in the guinea-pig ileum. Br J Pharmacol. 1977 Dec;61(4):541–549. doi: 10.1111/j.1476-5381.1977.tb07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY W. L. M., TALESNIK J. The role of acetylcholine in synaptic transmission at parasympathetic ganglia. J Physiol. 1953 Mar;119(4):455–469. doi: 10.1113/jphysiol.1953.sp004859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper C. M., Henderson G. Opiates and opioid peptides hyperpolarize locus coeruleus neurons in vitro. Science. 1980 Jul 18;209(4454):394–395. doi: 10.1126/science.7384811. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Volle R. L. Nicotinic, muscarinic and adrenergic receptors in a parasympathetic ganglion. J Pharmacol Exp Ther. 1979 Oct;211(1):252–256. [PubMed] [Google Scholar]
- Szerb J. C. Effect of low calcium and of oxotremorine on the kinetics of the evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus; comparison with morphine. Naunyn Schmiedebergs Arch Pharmacol. 1980 Mar;311(2):119–127. doi: 10.1007/BF00510250. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Morita K., North A. Opiates and clonidine prolong calcium-dependent after-hyperpolarizations. Nature. 1981 Nov 12;294(5837):162–163. doi: 10.1038/294162a0. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Heterogeneous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for mu- and delta-opiate receptors. Nature. 1982 Oct 21;299(5885):730–733. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Egan T. M., North R. A. Enkephalin opens potassium channels on mammalian central neurones. Nature. 1982 Sep 2;299(5878):74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Wouters W., van den Bercken J. Effects of met-enkephalin on slow synaptic inhibition in frog sympathetic ganglion. Neuropharmacology. 1980 Mar;19(3):237–243. doi: 10.1016/0028-3908(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Wouters W., van den Bercken J. Hypepolarisation and depression of slow synaptic inhibition by enkephalin in frog sympathetic ganglion. Nature. 1979 Jan 4;277(5691):53–54. doi: 10.1038/277053a0. [DOI] [PubMed] [Google Scholar]


