Abstract
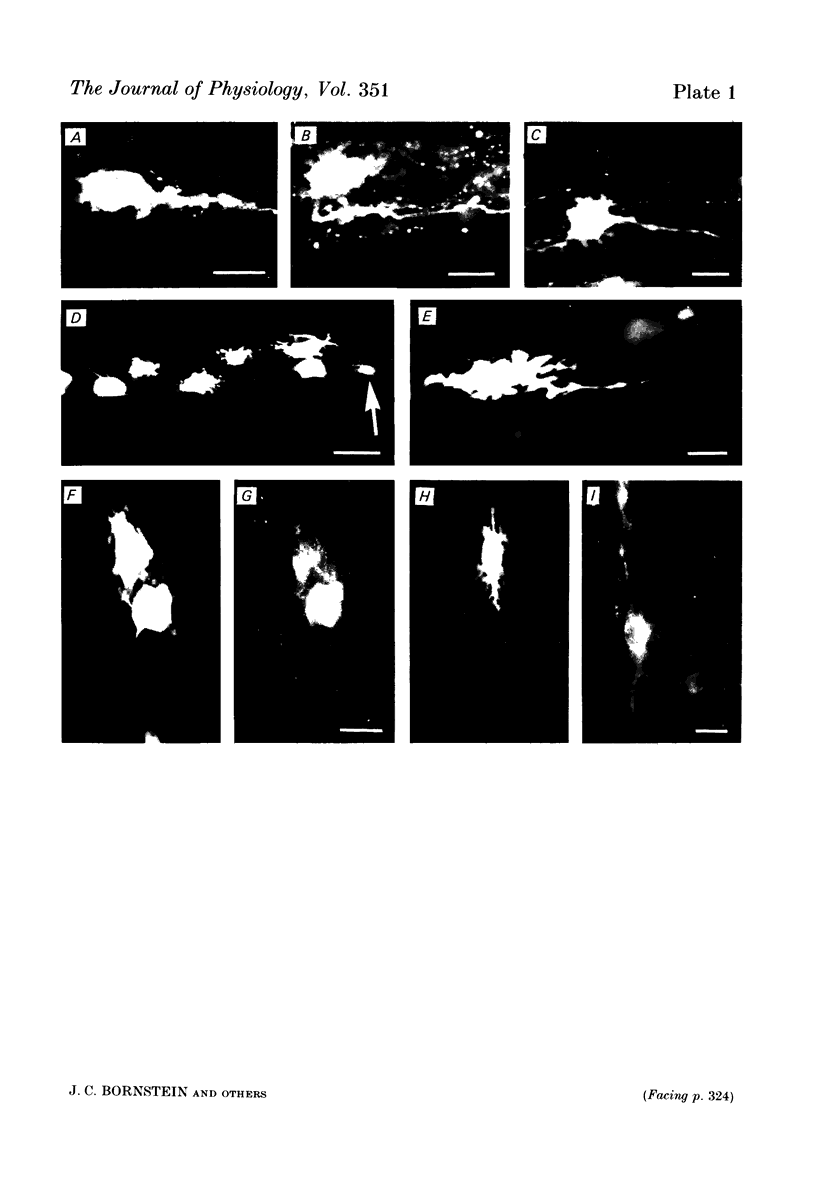
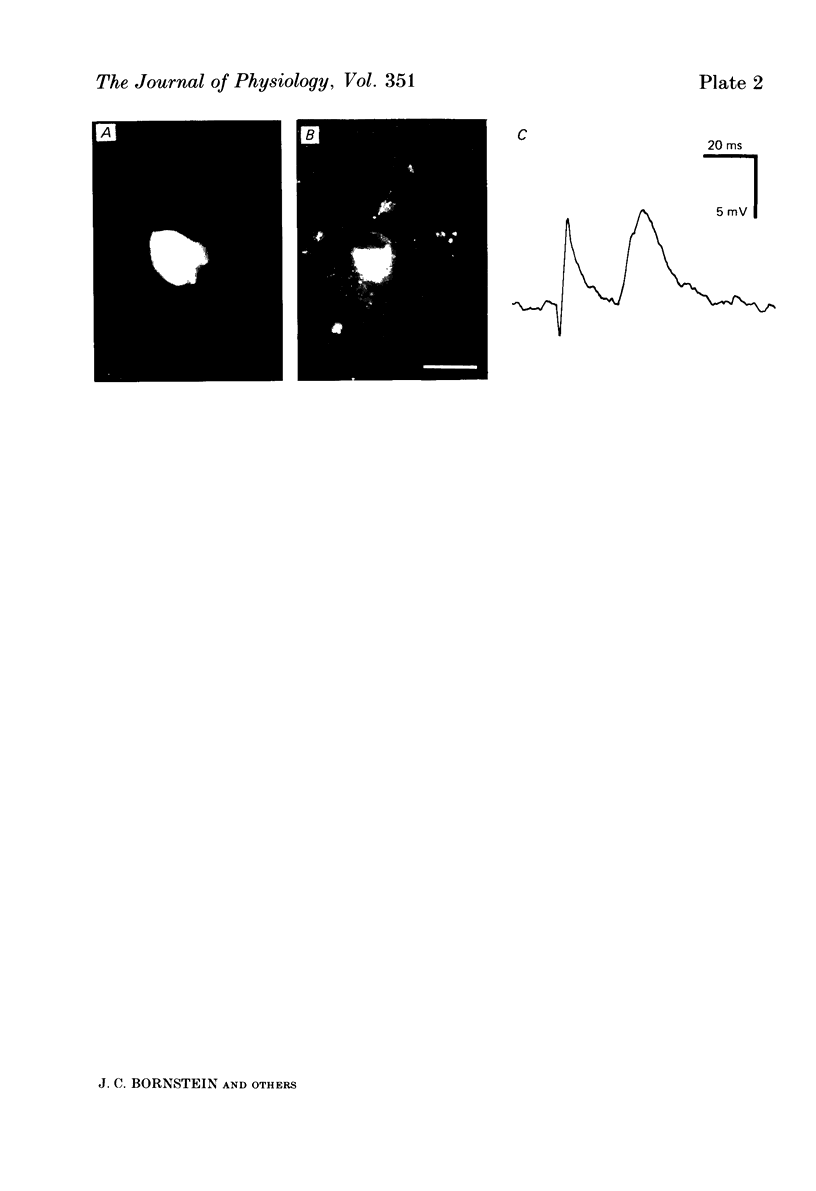
Intracellular injection of the fluorescent dye, Lucifer Yellow CH, revealed the shapes of neurones in the myenteric plexus of the guinea-pig ileum, and these shapes were correlated with the electrophysiological properties and enkephalin immunoreactivity of the neurones. A total of eighty-three neurones were filled using electrodes containing a 5% solution of the dye. Forty-six cells had many short processes and a single long process (Dogiel type 1) and twenty-four cells had essentially smooth somas and one to eight long processes (Dogiel type II). Thirteen cells could not be put into either group. Enkephalin-like immunoreactivity was detected in twenty-two of the forty-six Dogiel type I cells. Eighteen of these had club-like short processes. No other cells of the eighty-three showed enkephalin-like immunoreactivity. Electrodes filled with a 0.5% solution of Lucifer Yellow in 0.5 M-KCl were used to record from and simultaneously to inject dye into 240 neurones. Eighty-six nerve cells had a slow after-hyperpolarization following the action potential (AH cells) and forty-six nerve cells had no after-hyperpolarization but exhibited a fast excitatory synaptic potential (S cells). The other cells could not be unequivocally identified by their observed electrophysiological characteristics. Almost all S cells (forty-two of forty-six) were Dogiel type I, while eighty-two of the eighty-six AH cells were Dogiel type II. Fifty S cells (eight located geometrically, forty-two by dye injection) and ninety-one AH cells (twenty-six located geometrically, sixty-five by dye injection) were examined for enkephalin immunoreactivity. Fifteen of the S cells were reactive, whereas all of the AH cells were unreactive. It appears that prolonged impalements reduce immunoreactivity so that the proportion of reactive neurones in this series is an underestimate of the true proportion of S cells with enkephalin-like immunoreactivity. The results suggest that a substantial proportion of the S cells in myenteric ganglia contain enkephalin immunoreactivity while none of the AH cells do. The enkephalin neurones have a distinctive shape and are all Dogiel type I cells. AH cells are nearly always Dogiel type II.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock H. The strength-duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters. J Physiol. 1983 Aug;341:59–74. doi: 10.1113/jphysiol.1983.sp014792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu'Lock A. J., Vaillant C., Dockray G. J., Bu'Lock J. D. A rational approach to the fixation of peptidergic nerve cell bodies in the gut using parabenzoquinone. Histochemistry. 1982;74(1):49–55. doi: 10.1007/BF00495051. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., McKnight A. T., Kosterlitz H. W. Tetraethylammonium facilitates the stimulation-evoked loss of the enkephalins from the myenteric plexus of guinea-pig ileum. Proc R Soc Lond B Biol Sci. 1981 Oct 14;213(1191):171–182. doi: 10.1098/rspb.1981.0060. [DOI] [PubMed] [Google Scholar]
- Costa M., Buffa R., Furness J. B., Solcia E. Immunohistochemical localization of polypeptides in peripheral autonomic nerves using whole mount preparations. Histochemistry. 1980 Feb;65(2):157–165. doi: 10.1007/BF00493164. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. Neuronal peptides in the intestine. Br Med Bull. 1982 Sep;38(3):247–252. doi: 10.1093/oxfordjournals.bmb.a071768. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Miller R. J. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983 Apr;8(4):653–664. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkiss J. P., Lees G. M. Correlated electrophysiological and morphological characteristics of myenteric plexus neurones [proceedings]. J Physiol. 1978 Dec;285:19P–20P. [PubMed] [Google Scholar]
- Hodgkiss J. P., Lees G. M. Morphological studies of electrophysiologically-identified myenteric plexus neurons of the guinea-pig ileum. Neuroscience. 1983 Mar;8(3):593–608. doi: 10.1016/0306-4522(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Lees G. M., Gray M. J. A method for simultaneous visualization and electrophysiological recording of enteric neurones with intracellular fluorescent markers. Scand J Gastroenterol Suppl. 1982;71:169–170. [PubMed] [Google Scholar]
- Miller R. J., Chang K. J., Cooper B., Cuatrecasas P. Radioimmunoassay and characterization of enkephalins in rat tissues. J Biol Chem. 1978 Jan 25;253(2):531–538. [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A. Electrophysiology of the enteric nervous system. Neuroscience. 1982 Feb;7(2):315–325. doi: 10.1016/0306-4522(82)90269-x. [DOI] [PubMed] [Google Scholar]
- Orkand R. K., Nicholls J. G., Kuffler S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957 Mar;12(1):119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig M. M., Gascon P., Craviso G. L., Musacchio J. M. Endogenous opiate receptor ligand: electrically induced release in the guinea pig ileum. Science. 1977 Jan 28;195(4276):419–420. doi: 10.1126/science.188138. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Goldring S. Ionic determinants of membrane potential of cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973 Sep;36(5):855–868. doi: 10.1152/jn.1973.36.5.855. [DOI] [PubMed] [Google Scholar]
- Reaves T. A., Jr, Hayward J. N. Intracellular dye-marked enkephalin neurons in the magnocellular preoptic nucleus of the goldfish hypothalamus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6009–6011. doi: 10.1073/pnas.76.11.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J. F., Brown M., Elde R., Goldstein M., Said S. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience. 1980;5(4):689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Lucifer dyes--highly fluorescent dyes for biological tracing. Nature. 1981 Jul 2;292(5818):17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- Sundler F., Håkanson R., Leander S. Peptidergic nervous systems in the gut. Clin Gastroenterol. 1980 Sep;9(3):517–543. [PubMed] [Google Scholar]
- Waterfield A. A., Kosterlitz H. W. Stereospecific increase by narcotic antagonists of evoked acetylcholine output in guinea-pig ileum. Life Sci. 1975 Jun 15;16(12):1787–1792. doi: 10.1016/0024-3205(75)90275-1. [DOI] [PubMed] [Google Scholar]
- Waterfield A. A., Smokcum R. W., Hughes J., Kosterlitz H. W., Henderson G. In vitro pharmacology of the opioid peptides, enkephalins and endorphins. Eur J Pharmacol. 1977 May 15;43(2):107–116. doi: 10.1016/0014-2999(77)90123-6. [DOI] [PubMed] [Google Scholar]