Abstract
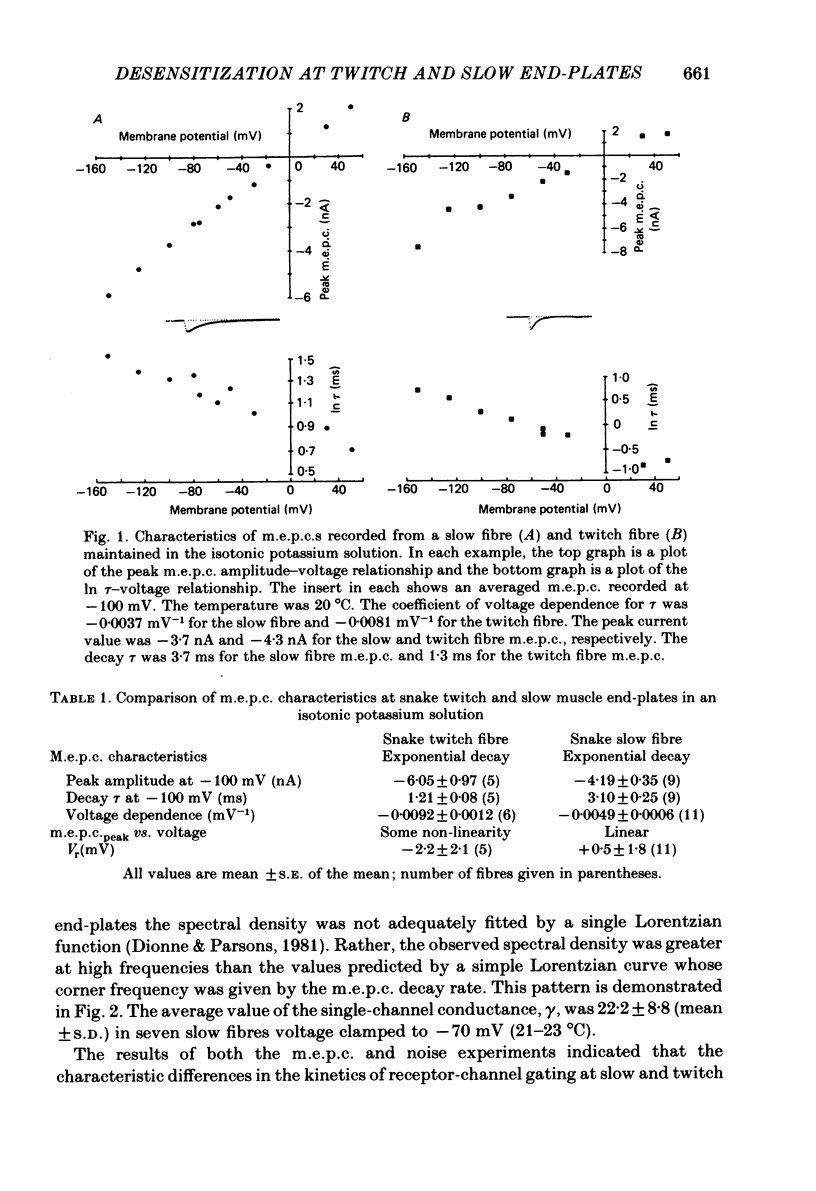
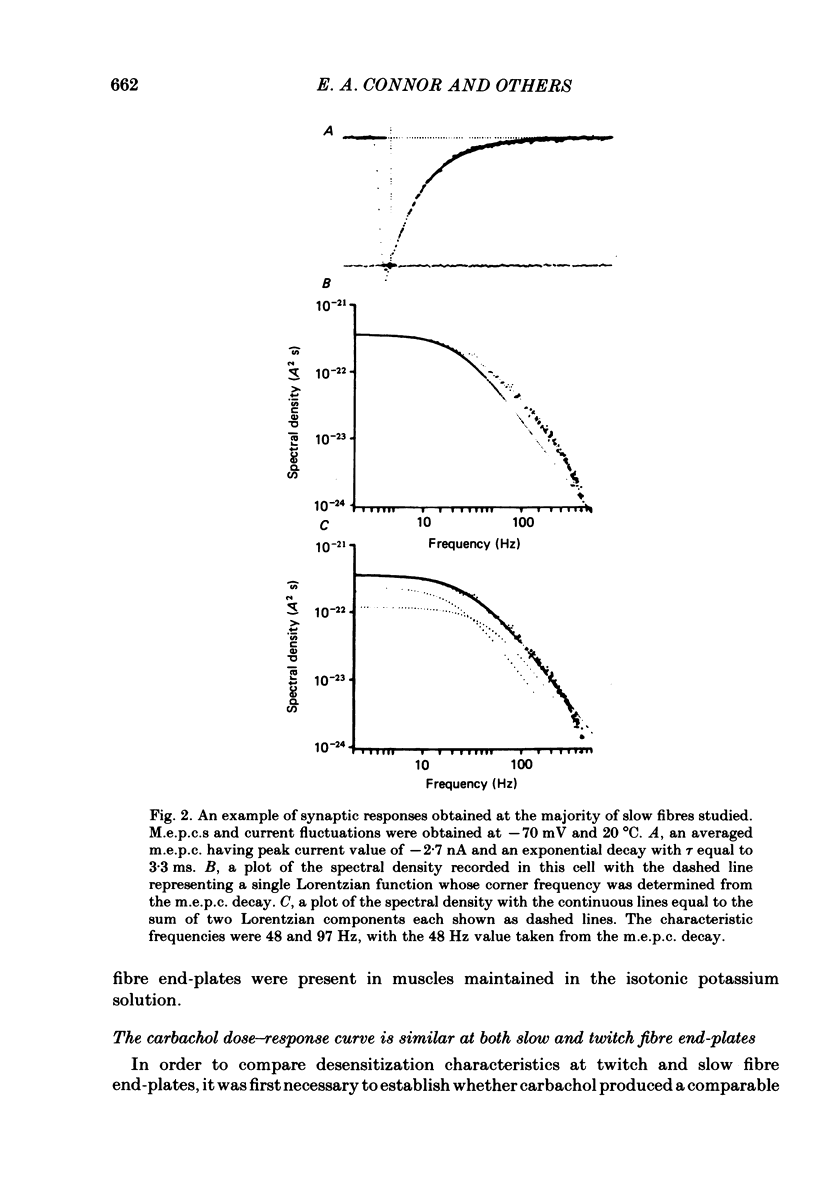
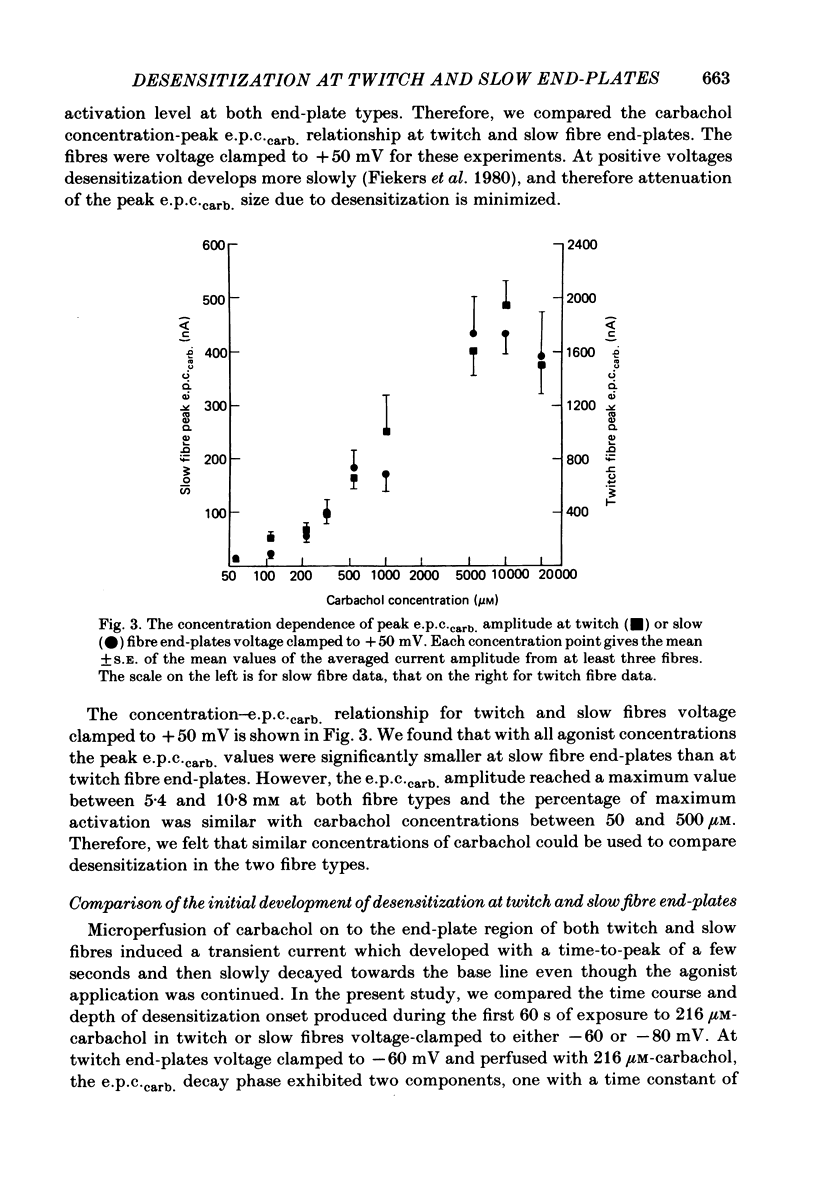
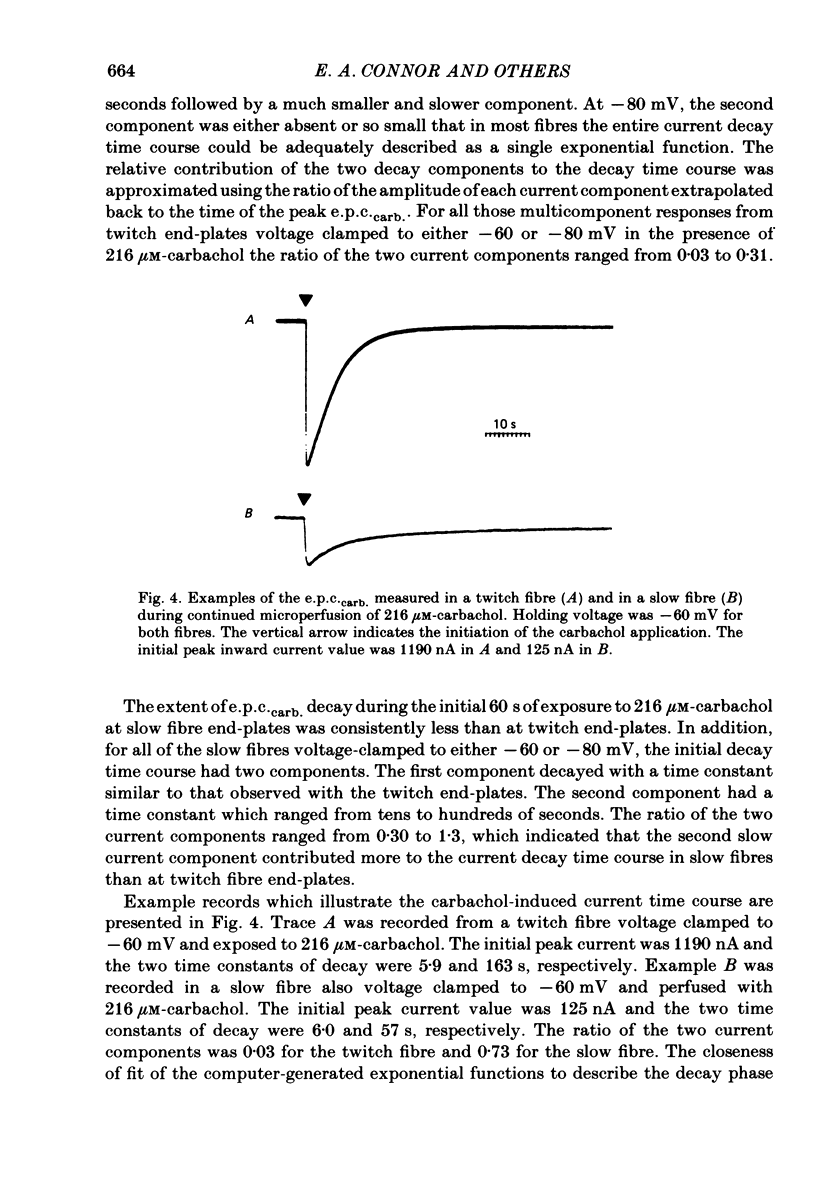
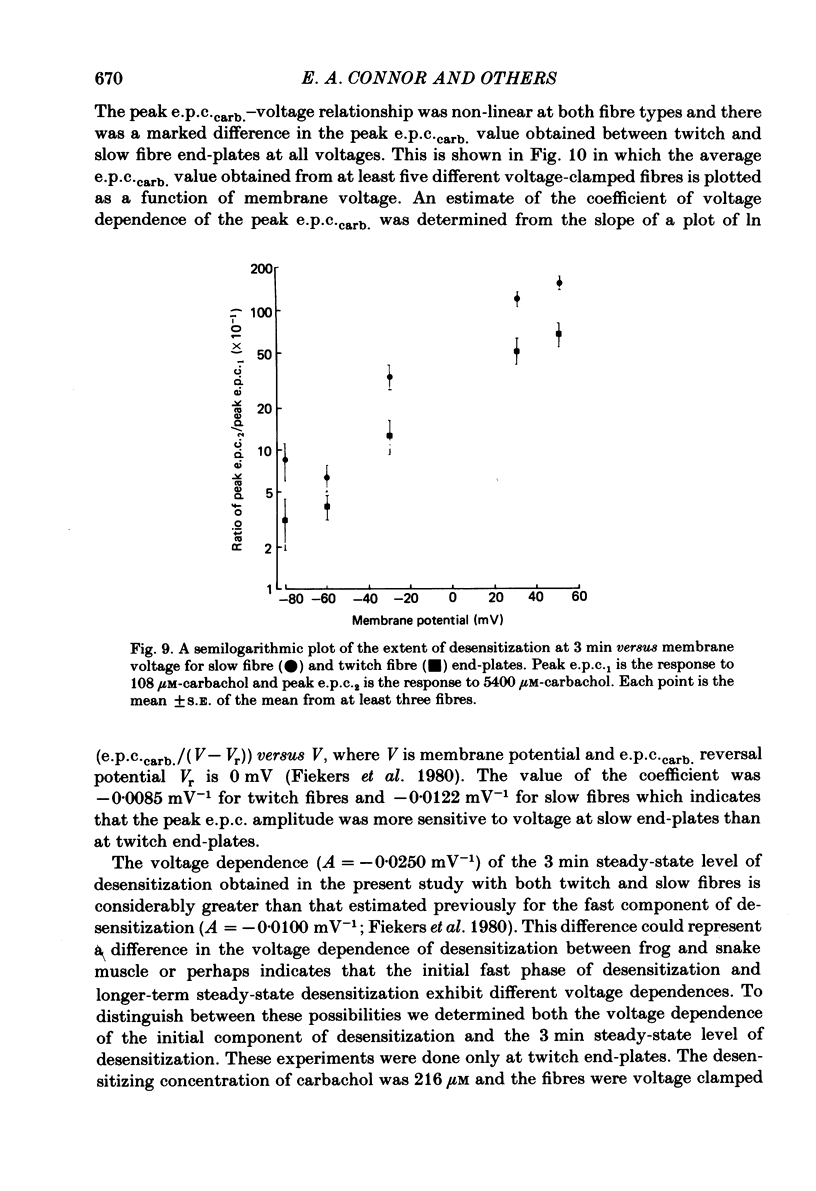
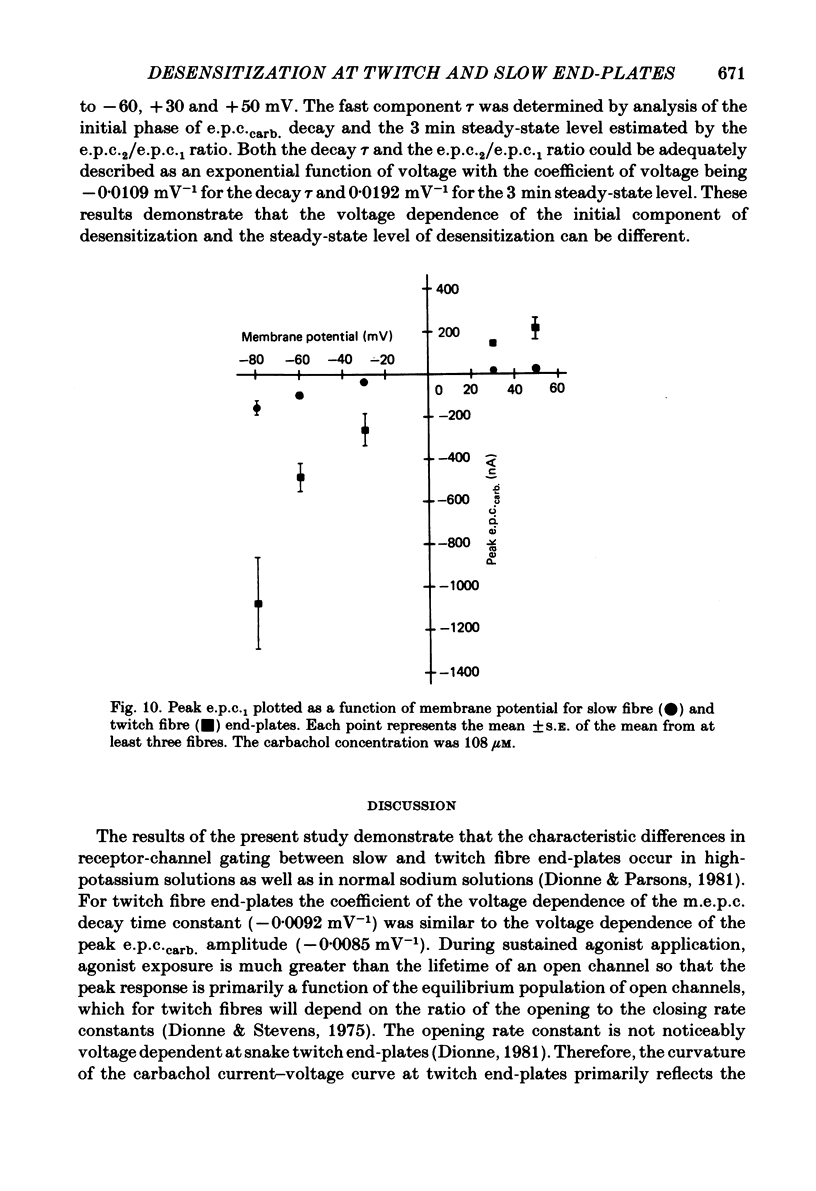
Characteristics of receptor-channel activation and desensitization have been compared at voltage-clamped snake slow and twitch fibre end-plates maintained in an isotonic potassium propionate solution. Miniature end-plate current (m.e.p.c.) decay was slower and less voltage dependent at slow fibre end-plates than at twitch fibre end-plates. The peak m.e.p.c. amplitude versus voltage relationship and reversal potential were similar at the two end-plate types. Acetylcholine-induced noise and m.e.p.c.s were recorded at slow fibre end-plates. At most slow fibres the spectral density was not adequately fitted by a single Lorentzian function. Rather, the observed spectral density was greater at high frequencies than the values predicted using the m.e.p.c. decay rate. The noise could be well described by the sum of two Lorentzian functions, one of which corresponded to a single Lorentzian function with the corner frequency determined by the m.e.p.c. decay rate. The shape of the carbachol concentration-peak end-plate current relationship was similar at both slow and twitch fibre end-plates. However, for all concentrations tested, the peak carbachol-induced end-plate current (e.p.c.carb.) value was markedly less at slow fibre end-plates than at twitch fibre end-plates. The onset of desensitization was determined using two methods. The first concerned analysis of the time course of decay of the e.p.c.carb. from a peak value during the sustained application of agonist. The second involved a double-perfusion technique in which a 'desensitizing' dose was applied for varying intervals before the application of a second 'test' dose of carbachol. With both methods the development of desensitization at both end-plate types was dependent on carbachol concentration and duration of exposure. At each end-plate type the time course of desensitization onset often exhibited two components; one with a time constant of seconds and a slower component having time constants in the range of tens to hundreds of seconds. The slope of the relationship between carbachol concentration and equilibrium desensitization at slow and twitch fibre end-plates was close to two, suggesting that two molecules of agonist are probably bound during the development of desensitization. However, for all concentrations tested, desensitization developed more rapidly and to a greater extent at twitch fibre end-plates than at slow fibre end-plates. The voltage dependence of the 3 min steady-state desensitization produced by 108 microM-carbachol was very similar (approximately -0.0250 mV-1) at both fibre types.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesnut T. J. Two-component desensitization at the neuromuscular junction of the frog. J Physiol. 1983 Mar;336:229–241. doi: 10.1113/jphysiol.1983.sp014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBassio W. A., Parsons R. L., Schnitzler R. M. Effect of ionophore X-537A on desensitization rate and tension development in potassium-depolarized muscle fibres. Br J Pharmacol. 1976 Aug;57(4):565–571. doi: 10.1111/j.1476-5381.1976.tb10386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Parsons R. L. Characteristics of the acetylcholine-operated channel at twitch and slow fibre neuromuscular junctions of the garter snake. J Physiol. 1981 Jan;310:145–158. doi: 10.1113/jphysiol.1981.sp013541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E. The kinetics of slow muscle acetylcholine-operated channels in the garter snake. J Physiol. 1981 Jan;310:159–190. doi: 10.1113/jphysiol.1981.sp013542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. The effect of calcium on the desensitization of membrane receptors at the neuromuscular junction. J Gen Physiol. 1966 May;49(5):963–976. doi: 10.1085/jgp.49.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R. L., Nastuk W. L. Activation of contractile system in depolarized skeletal muscle fibers. Am J Physiol. 1969 Aug;217(2):364–369. doi: 10.1152/ajplegacy.1969.217.2.364. [DOI] [PubMed] [Google Scholar]
- Parsons R. L., Schnitzler R. M., Cochrane D. E. Inhibition of end-plate desensitization by sodium. Am J Physiol. 1974 Jul;227(1):96–100. doi: 10.1152/ajplegacy.1974.227.1.96. [DOI] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization onset and recovery at the potassium-depolarized frog neuromuscular junction are voltage sensitive. J Gen Physiol. 1978 Mar;71(3):285–299. doi: 10.1085/jgp.71.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]


