Abstract
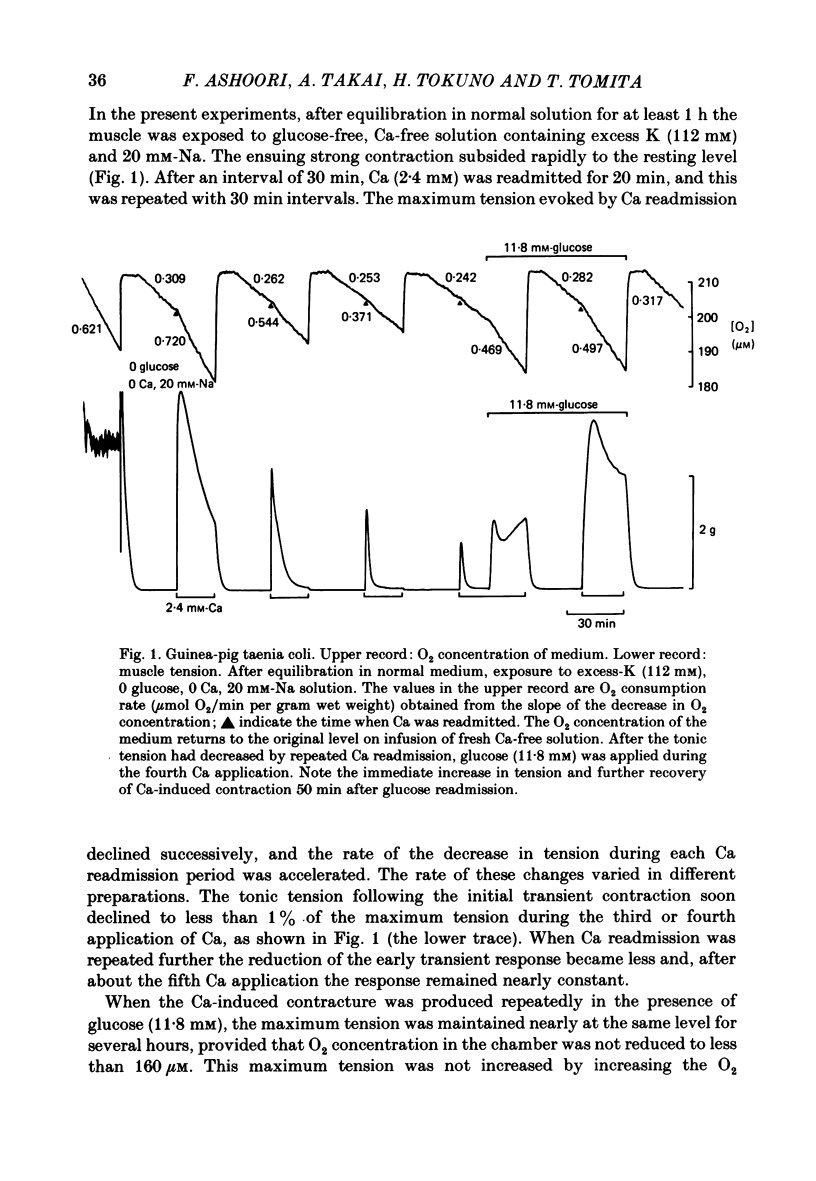
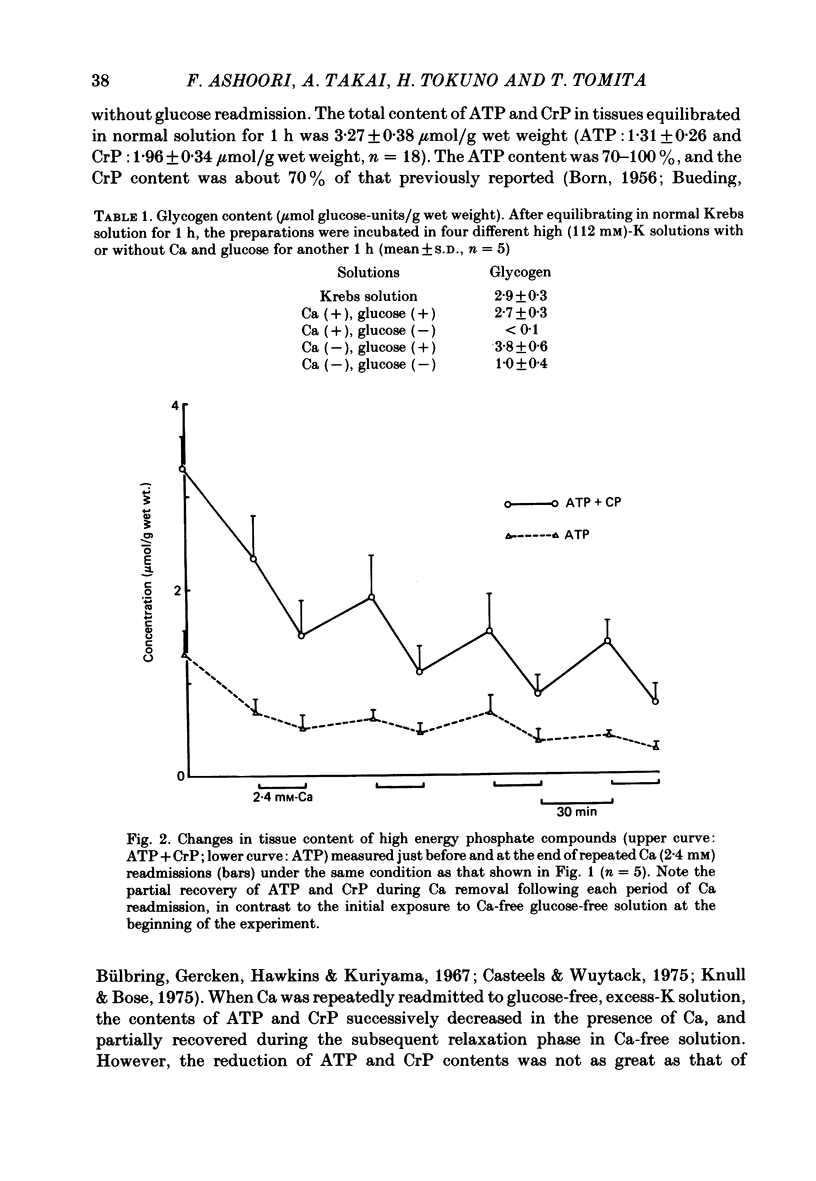
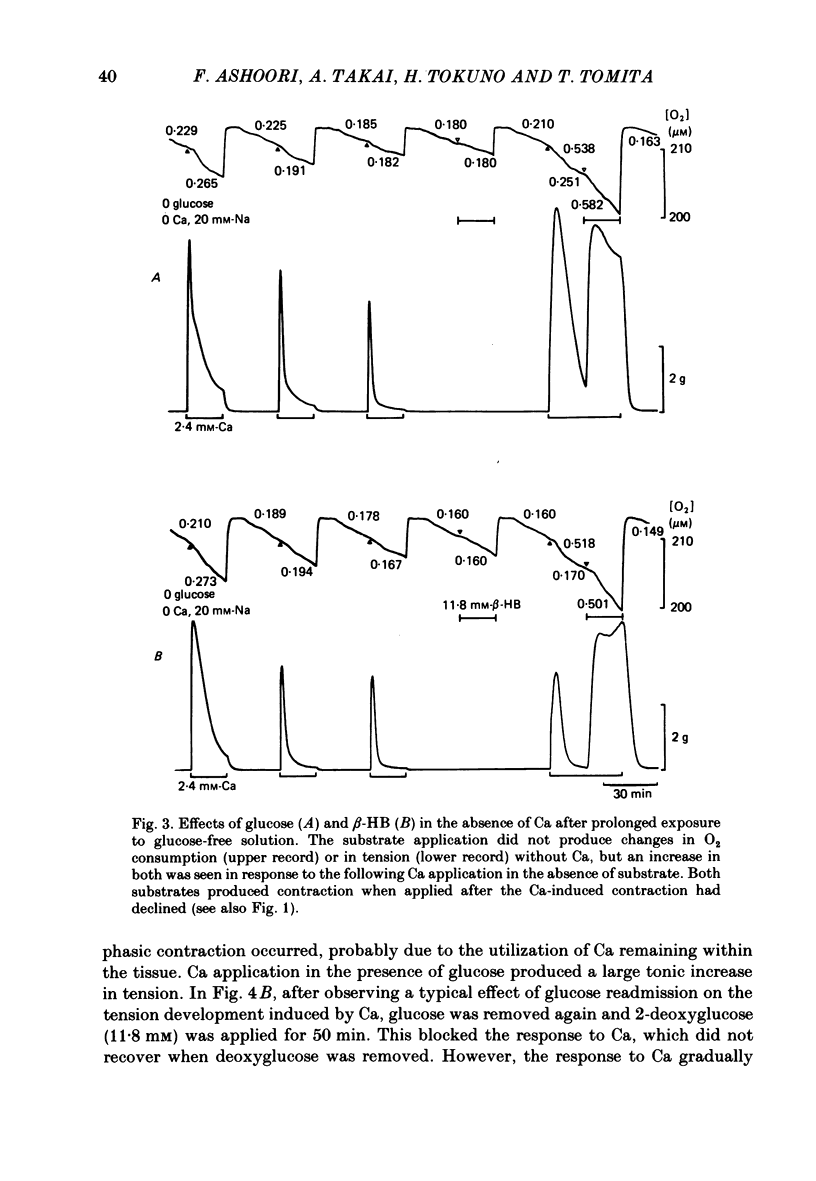
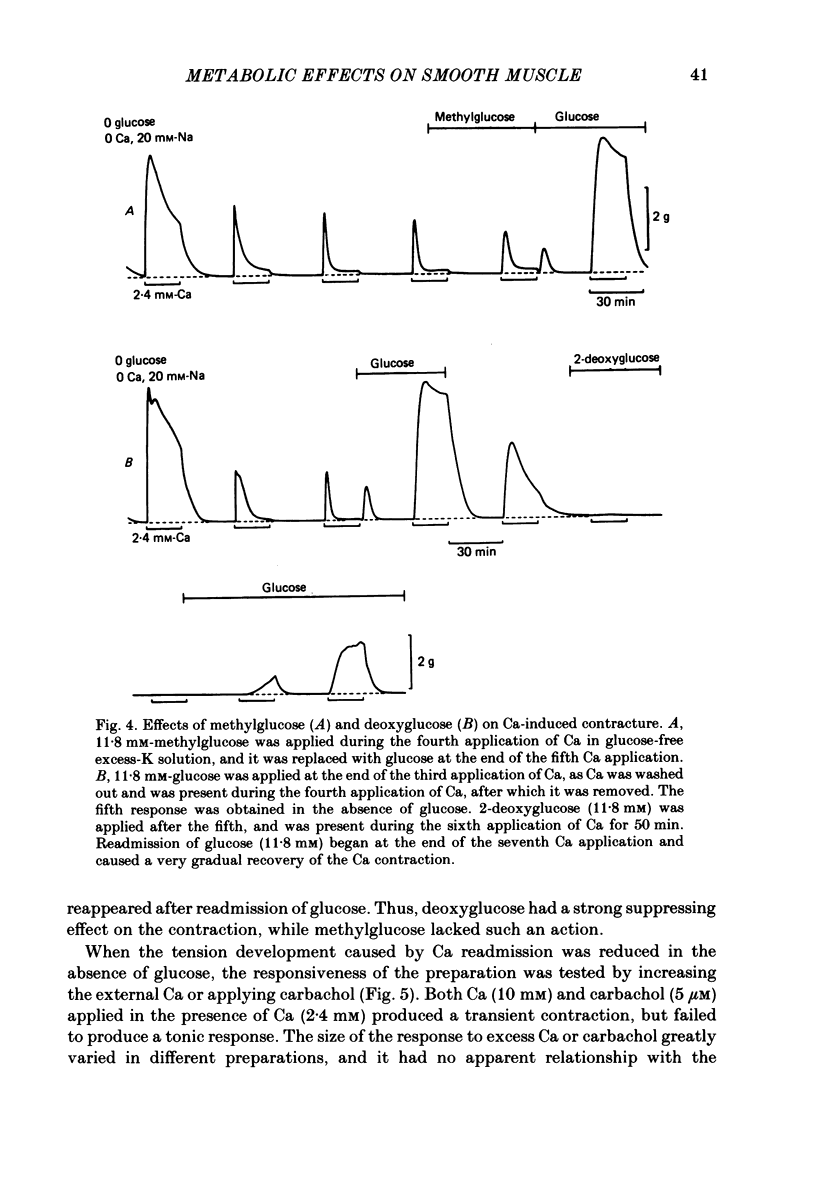
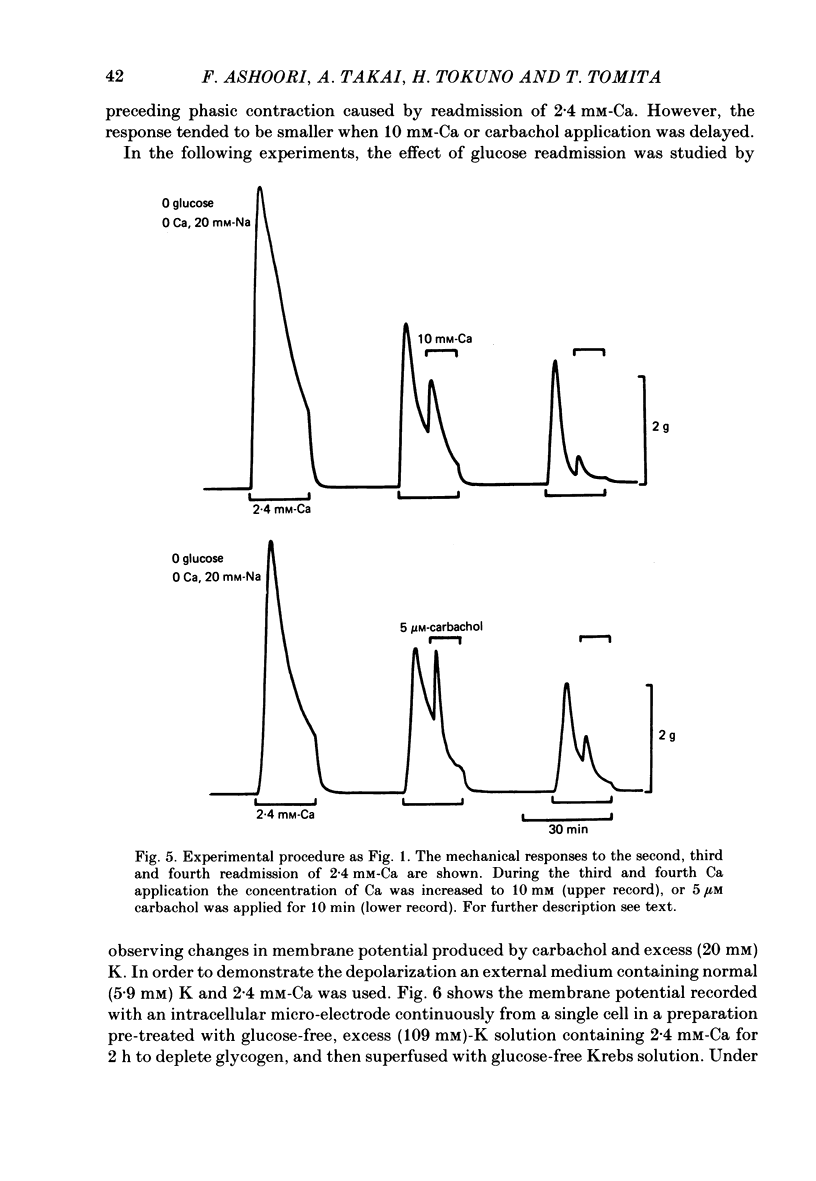
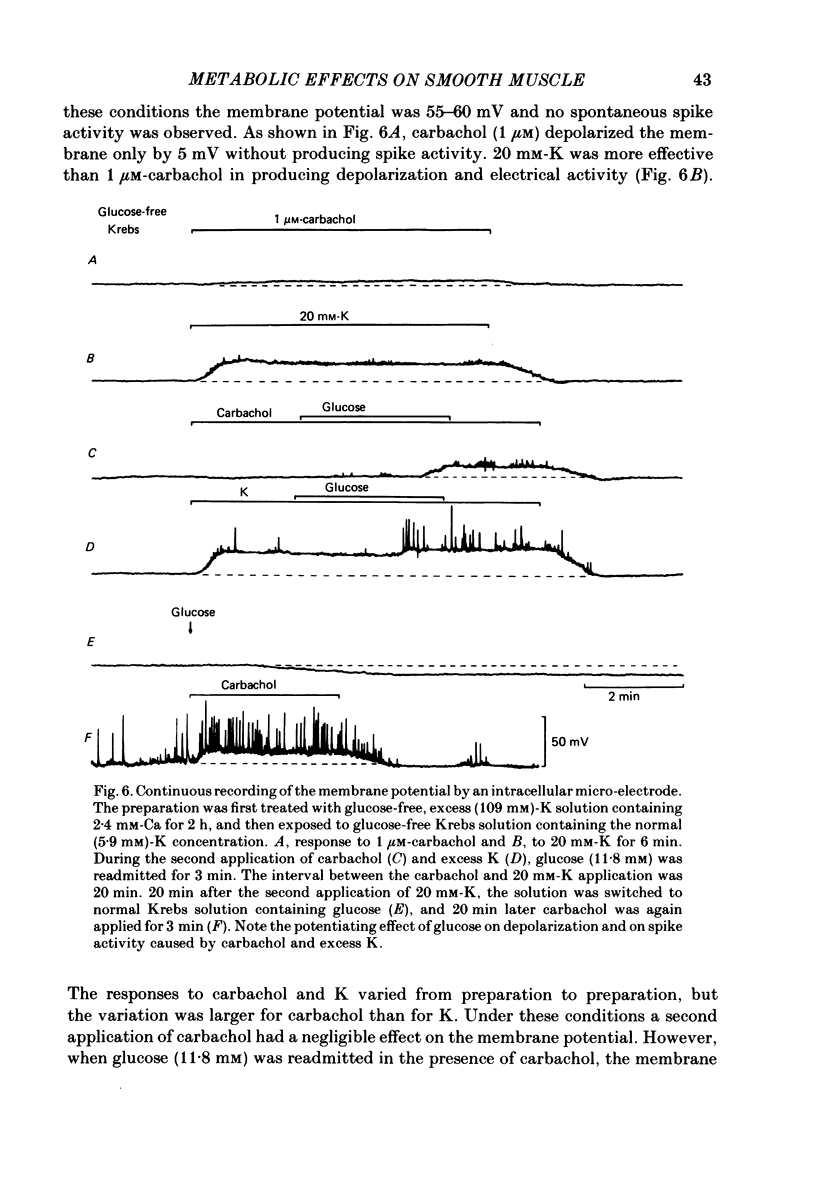
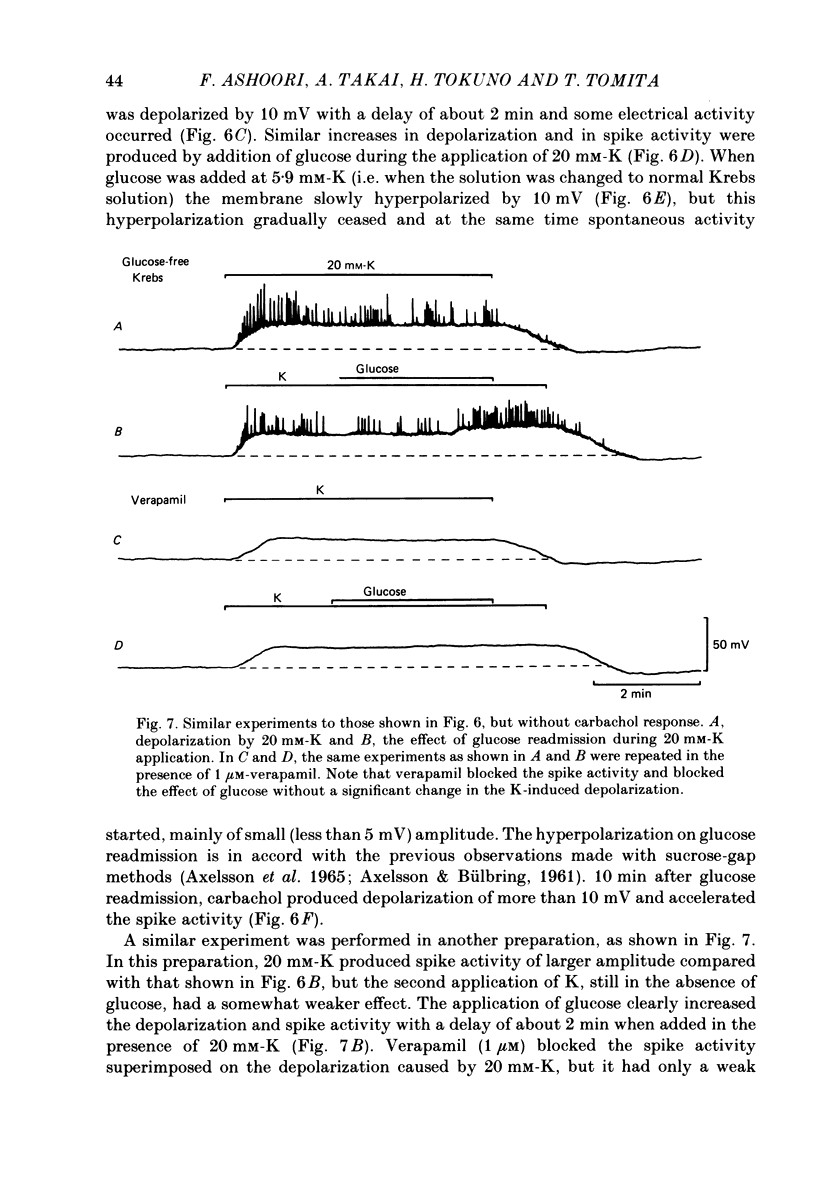
The effects of removal and readmission of substrates on the K contracture were investigated in the guinea-pig taenia coli. When, after exposure to excess K in Ca-free and glucose-free medium, the readmission and removal of 2.4 mM-Ca were repeated at regular intervals, the Ca-induced contractions decreased progressively. The decrease was more marked in the late than in the early part of the tension response. The rate of O2 consumption decreased when the normal medium was replaced by glucose-free, Ca-free, excess-K solution, but substantially recovered following Ca readmission. ATP and creatine phosphate contents decreased during the Ca-induced contraction, but recovered partially during the subsequent relaxation in Ca-free solution. The effects of glucose removal were rapidly reversed when glucose or beta-hydroxybutyrate (beta-HB) were readmitted to the bathing solution. In the absence of Ca, readmission and removal of the substrates produced an insignificant change in O2 consumption, but the next Ca contraction was potentiated, the effect being stronger with glucose than beta-HB. When the tonic contraction evoked by 2.4 mM-Ca readmission had been abolished in glucose-free, high-K solution, a rise of the external Ca concentration to 10 mM, or 5 microM-carbachol, still produced a transient contraction. This suggests that the tonic contraction has disappeared partially because of diminished Ca influx. In glycogen-depleted preparations, the depolarization caused by carbachol, or by 20 mM-K, was increased and spike discharge initiated when glucose was readmitted.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., BUEDING E., BULBRING E. The inhibitory action of adrenaline on intestinal smooth muscle in relation to its action on phosphorylase activity. J Physiol. 1961 Apr;156:357–374. doi: 10.1113/jphysiol.1961.sp006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELSSON J., BULBRING E. Metabolic factors affecting the electrical activity of intestinal smooth muscle. J Physiol. 1961 Apr;156:344–356. doi: 10.1113/jphysiol.1961.sp006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist H. J. Studies of monosaccharide permeability of arterial tissue and intestinal smooth muscle; effects of insulin. Acta Physiol Scand. 1971 Oct;83(2):247–256. doi: 10.1111/j.1748-1716.1971.tb05074.x. [DOI] [PubMed] [Google Scholar]
- BORN G. V. The relation between the tension and the high-energy phosphate content of smooth muscle. J Physiol. 1956 Mar 28;131(3):704–711. doi: 10.1113/jphysiol.1956.sp005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUEDING E., HAWKINS J. T. ENZYMIC DEGRADATION AND MICRODETERMINATION OF GLYCOGEN. Anal Biochem. 1964 Jan;7:26–36. doi: 10.1016/0003-2697(64)90116-2. [DOI] [PubMed] [Google Scholar]
- Bueding E., Bülbring E., Gercken G., Hawkins J. T., Kuriyama H. The effect of adrenaline on the adenosine otriphosphate and creatine phosphate content of intestinal smooth muscle. J Physiol. 1967 Nov;193(1):187–212. doi: 10.1113/jphysiol.1967.sp008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Golenhofen K. Oxygen consumption by the isolated smooth muscle of guinea-pig taenia coli. J Physiol. 1967 Nov;193(1):213–224. doi: 10.1113/jphysiol.1967.sp008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Wuytack F. Aerobic and anaerobic metabolism in smooth muscle cells of taenia coli in relation to active ion transport. J Physiol. 1975 Sep;250(2):203–220. doi: 10.1113/jphysiol.1975.sp011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand P., Johansson B., Norberg K. Mechanical, electrical, and biochemical effects of hypoxia and substrate removal on spontaneously active vascular smooth muscle. Acta Physiol Scand. 1977 May;100(1):69–83. doi: 10.1111/j.1748-1716.1977.tb05923.x. [DOI] [PubMed] [Google Scholar]
- Hellstrand P. Oxygen consumption and lactate production of the rat portal vein in relation to its contractile activity. Acta Physiol Scand. 1977 May;100(1):91–106. doi: 10.1111/j.1748-1716.1977.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Imai S., Takeda K. Actions of calcium and certain multivalent cations on potassium contracture of guinea-pig's taenia coli. J Physiol. 1967 May;190(1):155–169. doi: 10.1113/jphysiol.1967.sp008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Ganeshanandan S. S., Ikeda M., Urakawa N. Changes in tension Ca movement and metabolism of guinea pig taenia coli in varying concentrations of external Na and K. Jpn J Pharmacol. 1969 Dec;19(4):569–577. doi: 10.1254/jjp.19.569. [DOI] [PubMed] [Google Scholar]
- Karaki H., Ikeda M., Urakawa N. Movements of calcium during tension development induced by barium and high-potassium in guinea pig taenia coli. Jpn J Pharmacol. 1969 Jun;19(2):291–299. doi: 10.1254/jjp.19.291. [DOI] [PubMed] [Google Scholar]
- Karaki H., Suzuki T., Urakawa N., Ishida Y., Shibata S. High K+,Na+-deficient solution inhibits tension, O2 consumption, and ATP synthesis in smooth muscle. Jpn J Pharmacol. 1982 Aug;32(4):727–733. doi: 10.1254/jjp.32.727. [DOI] [PubMed] [Google Scholar]
- Knull H. R., Bose D. Reversibility of mechanical and biochemical changes in smooth muscle due to anoxia and substrate depletion. Am J Physiol. 1975 Aug;229(2):329–333. doi: 10.1152/ajplegacy.1975.229.2.329. [DOI] [PubMed] [Google Scholar]
- PFAFFMAN M., URAKAWA N., HOLLAND W. C. ROLE OF METABOLISM IN K-INDUCED TENSION CHANGES IN GUINEA PIG TAENIA COLI. Am J Physiol. 1965 Jun;208:1203–1205. doi: 10.1152/ajplegacy.1965.208.6.1203. [DOI] [PubMed] [Google Scholar]
- Paul R. J. Coordination of metabolism and contractility in vascular smooth muscle. Fed Proc. 1983 Jan;42(1):62–66. [PubMed] [Google Scholar]
- Paul R. J., Peterson J. W. Relation between length, isometric force, and O2 consumption rate in vascular smooth muscle. Am J Physiol. 1975 Mar;228(3):915–922. doi: 10.1152/ajplegacy.1975.228.3.915. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., TOTTER J. R. Firefly luminescence in the study of energy transfer mechanisms. I. Substrate and enzyme determination. Arch Biochem Biophys. 1952 Sep;40(1):28–41. doi: 10.1016/0003-9861(52)90070-2. [DOI] [PubMed] [Google Scholar]
- Saito Y., Sakai Y., Ikeda M., Urakawa N. Oxygen consumption during potassium-induced contracture in guinea pig taenia coli. Jpn J Pharmacol. 1968 Sep;18(3):321–331. doi: 10.1254/jjp.18.321. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Skoog C. M. Tracheal smooth muscle and rate of oxygen uptake. Am J Physiol. 1974 Jun;226(6):1462–1467. doi: 10.1152/ajplegacy.1974.226.6.1462. [DOI] [PubMed] [Google Scholar]
- URAKAWA N., HOLLAND W. C. CA45 UPTAKE AND TISSUE CALCIUM IN K-INDUCED PHASIC AND TONIC CONTRACTION IN TAENIA COLI. Am J Physiol. 1964 Oct;207:873–876. doi: 10.1152/ajplegacy.1964.207.4.873. [DOI] [PubMed] [Google Scholar]
- Urakawa N., Ikeda M., Saito Y., Saki Y. Effects of calcium depletion on oxygen consumption in guinea pig taenia coli. Jpn J Pharmacol. 1968 Dec;18(4):500–508. doi: 10.1254/jjp.18.500. [DOI] [PubMed] [Google Scholar]


