Abstract
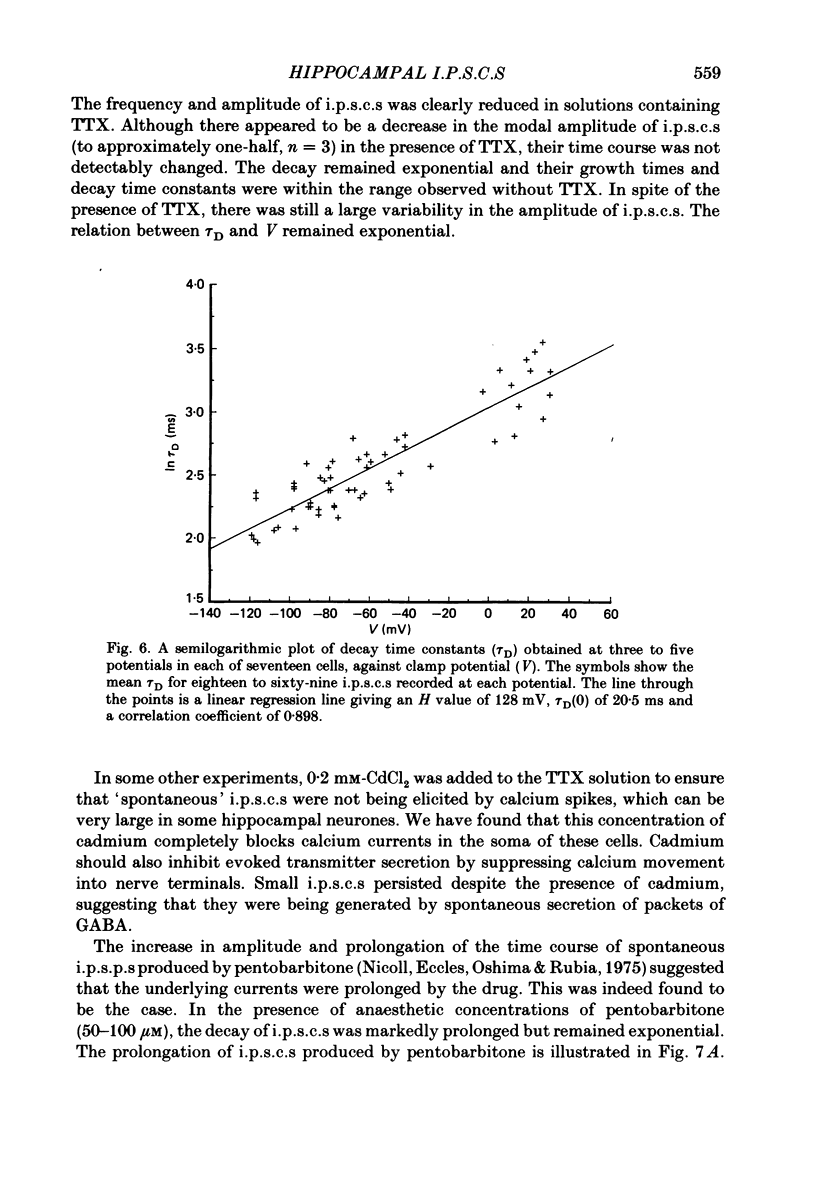
Spontaneous synaptic currents were recorded in voltage-clamped CA1 neurones in rat hippocampal slices at room temperature (21-25 degrees C). The currents, which could be seen at the resting membrane potential only when cells were loaded with chloride ions, were blocked by bicuculline. It was concluded that they were inhibitory post-synaptic currents (i.p.s.c.s) generated by the opening of chloride-selective channels activated by gamma-aminobutyric acid (GABA). In twenty-nine cells, ten to forty-five i.p.s.c.s were recorded at a potential between -75 and -85 mV. In every cell, i.p.s.c.s varied widely in amplitude with an average coefficient of variation of 28.7%. The mean amplitude varied from 0.22 to 1.16 nA with an average of 0.52 nA. Reversed (outward) currents could be recorded from cells voltage clamped at positive potentials. Null potentials varied between -20 and +20 mV, the variability being attributed to differences in chloride loading. Most currents had a rapid growth phase with a mean growth time (20-80% of peak) of 1.1 ms followed by a slower decay phase. The decay phase was exponential with a single time constant. The average decay time constant (tau D) ranged from 8.3 to 16.2 ms with a mean value of 11.0 ms. The rate of decay of currents was affected by membrane potential. tau D decreased exponentially with hyperpolarization in the range from +40 to -120 mV with an average volt constant (H value) of 146 +/- 9.6 mV (mean +/- 1 S.E. of mean, n = 17). The mean value of tau D at 0 mV was 19 ms. In some cells, growth times also decreased with hyperpolarization. The decay of currents was faster at higher temperatures but remained exponential. At 32 degrees C, the average tau D at 0 mV was 8.3 ms (n = 5) giving a Q10 value of 3.3 for the decay time constant at 0 mV. The frequency and mean amplitude of i.p.s.c.s were reduced by tetrodotoxin (TTX) or cadmium, indicating that many of the currents were generated by action potentials in presynaptic terminals. The spontaneous 'miniatures' remaining had the same time course and voltage sensitivity as currents recorded in normal solutions. Pentobarbitone (50-100 microM) greatly prolonged the decay of i.p.s.c.s but had no discernible effect on their amplitude or growth phase.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. LOCATION OF POSTSYNAPTIC INHIBITORY SYNAPSES ON HIPPOCAMPAL PYRAMIDS. J Neurophysiol. 1964 Jul;27:592–607. doi: 10.1152/jn.1964.27.4.592. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Banks F. W. Voltage clamp analysis of inhibitory synaptic action in crayfish stretch receptor neurons. Fed Proc. 1981 Sep;40(11):2637–2641. [PubMed] [Google Scholar]
- Aickin C. C., Deisz R. A. Pentobarbitone interference with inhibitory synaptic transmission in crayfish stretch receptor neurones. J Physiol. 1981 Jun;315:175–187. doi: 10.1113/jphysiol.1981.sp013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980 Oct 27;200(1):195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N. Phenobarbitone modulation of postsynaptic GABA receptor function on cultured mammalian neurons. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):319–327. doi: 10.1098/rspb.1979.0108. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Wong R. K., Prince D. A. Spontaneous miniature synaptic potentials in hippocampal neurons. Brain Res. 1979 Nov 9;177(1):194–199. doi: 10.1016/0006-8993(79)90931-4. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R. Junctional and extrajunctional membrane channels activated by GABA in locust muscle fibres. Proc R Soc Lond B Biol Sci. 1981 Mar 27;211(1185):527–535. doi: 10.1098/rspb.1981.0021. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R. Properties of miniature excitatory junctional currents at the locust nerve-muscle junction. J Physiol. 1982 May;326:527–551. doi: 10.1113/jphysiol.1982.sp014210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Felix D., McLellan H. GABA and hippocampal inhibition. Br J Pharmacol. 1970 Dec;40(4):881–883. doi: 10.1111/j.1476-5381.1970.tb10663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Voltage dependence of amplitude and time course of inhibitory synaptic current in crayfish muscle. Pflugers Arch. 1977 Oct 19;371(1-2):167–174. doi: 10.1007/BF00580786. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Single-shot channel activation accounts for duration of inhibitory postsynaptic potentials in a central neuron. Science. 1980 May 9;208(4444):612–615. doi: 10.1126/science.6245449. [DOI] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Transmission at a central inhibitory synapse. I. Magnitude of unitary postsynaptic conductance change and kinetics of channel activation. J Neurophysiol. 1982 Sep;48(3):654–678. doi: 10.1152/jn.1982.48.3.654. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Schultz S. G. Some properties of KCl-filled microelectrodes: correlation of potassium "leakage" with tip resistance. J Membr Biol. 1981;62(3):239–244. doi: 10.1007/BF01998169. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Martin A. R. Inhibitory conductance changes at synapses in the lamprey brainstem. Science. 1983 Jul 1;221(4605):85–87. doi: 10.1126/science.6857271. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Richards C. D. Barbiturate potentiation of hippocampal i.p.s.p.s is not mediated by blockade of GABA uptake [proceedings]. J Physiol. 1977 Jul;269(1):42P–44P. [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeda F. J., Hablitz J. J., Johnston D. Antagonism of GABA-mediated responses by d-tubocurarine in hippocampal neurons. J Neurophysiol. 1982 Sep;48(3):622–632. doi: 10.1152/jn.1982.48.3.622. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. An analysis of the inhibitory post-synaptic current in the voltage-clamped crayfish muscle. J Physiol. 1979 Jan;286:265–282. doi: 10.1113/jphysiol.1979.sp012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. A barbiturate induced intensification of the inhibitory potential in slices of guinea-pig olfactory cortex. J Physiol. 1978 Feb;275:559–566. doi: 10.1113/jphysiol.1978.sp012208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Smith A. D., Nunzi M. G., Gorio A., Takagi H., Wu J. Y. Glutamate decarboxylase immunoreactivity in the hippocampus of the cat: distribution of immunoreactive synaptic terminals with special reference to the axon initial segment of pyramidal neurons. J Neurosci. 1983 Jul;3(7):1450–1468. doi: 10.1523/JNEUROSCI.03-07-01450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. J., Gribkoff V. K., Cotman C. W., Lynch G. S. GDEE antagonism of iontophoretic amino acid excitations in the intact hippocampus and in the hippocampal slice preparation. Brain Res. 1976 Apr 9;105(3):471–481. doi: 10.1016/0006-8993(76)90594-1. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Wolf P., Haas H. L. Effects of diazepines and barbiturates on hippocampal recurrent inhibition. Naunyn Schmiedebergs Arch Pharmacol. 1977 Oct;299(3):211–218. doi: 10.1007/BF00500313. [DOI] [PubMed] [Google Scholar]


