Abstract
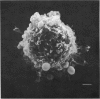
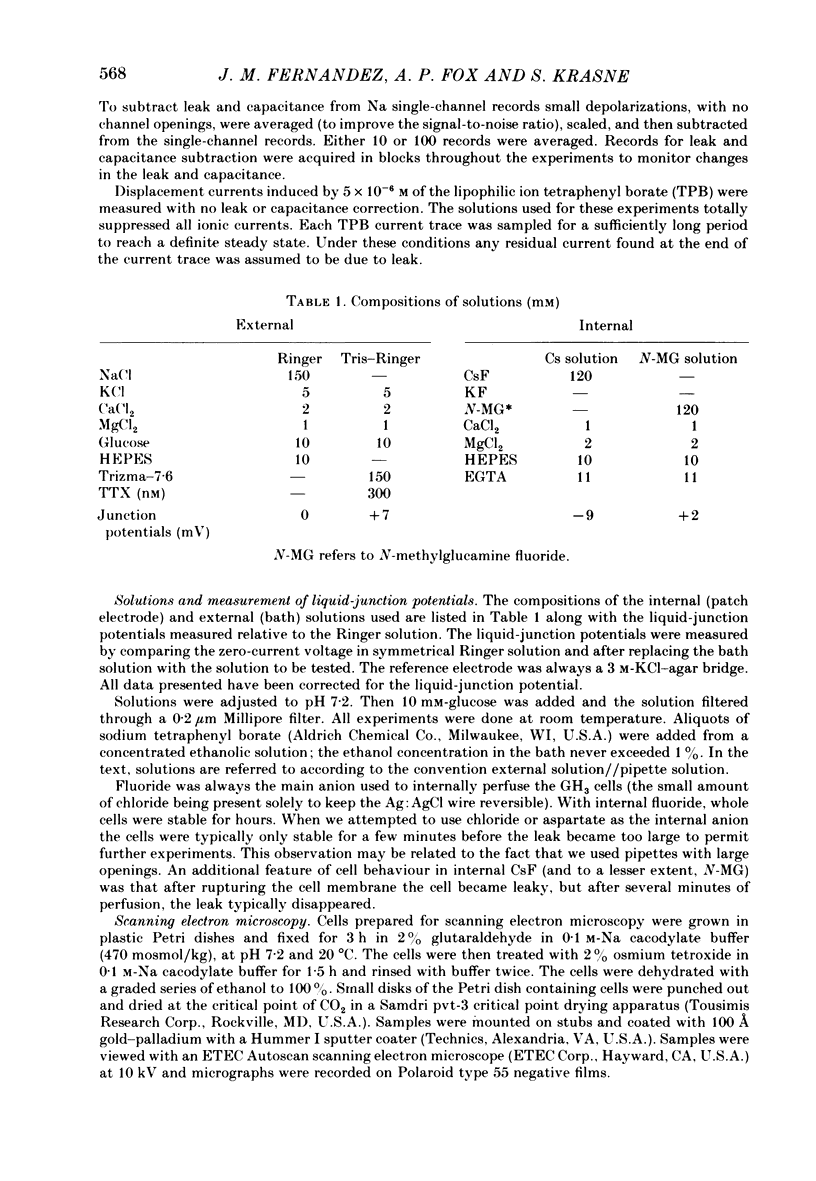
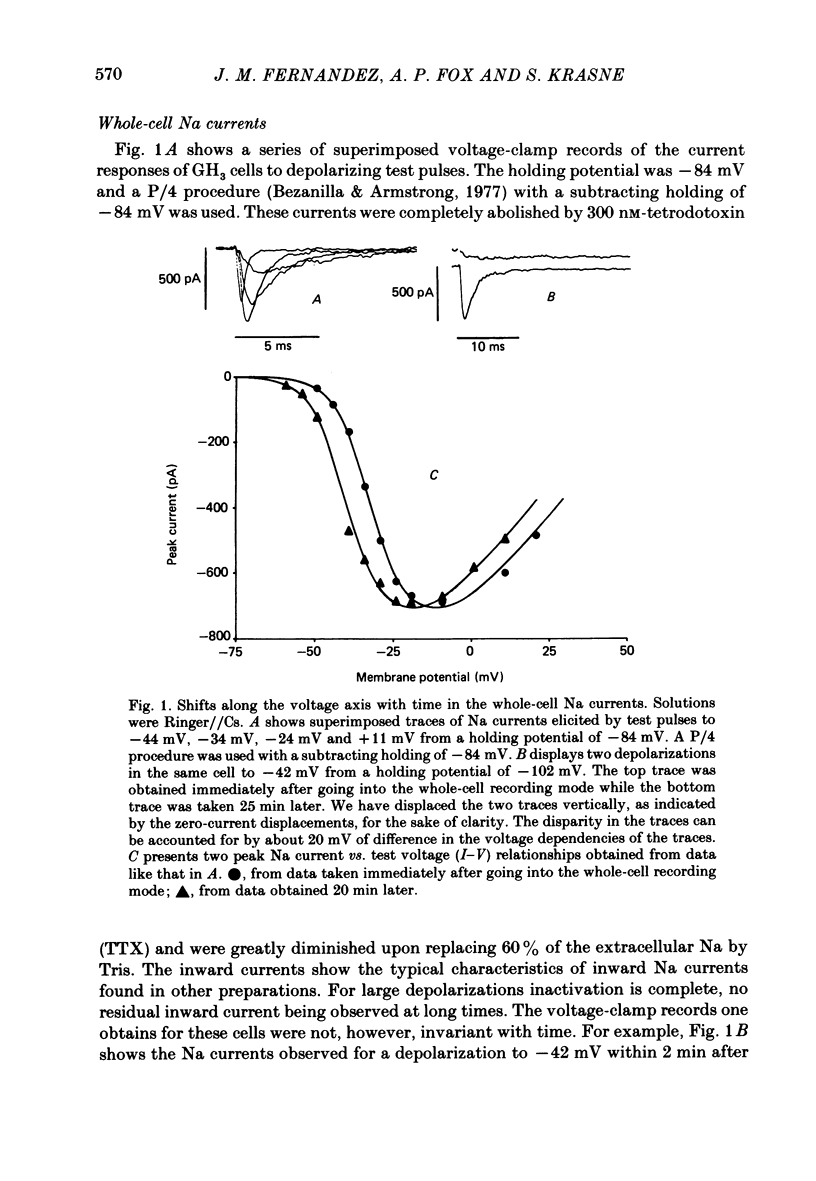
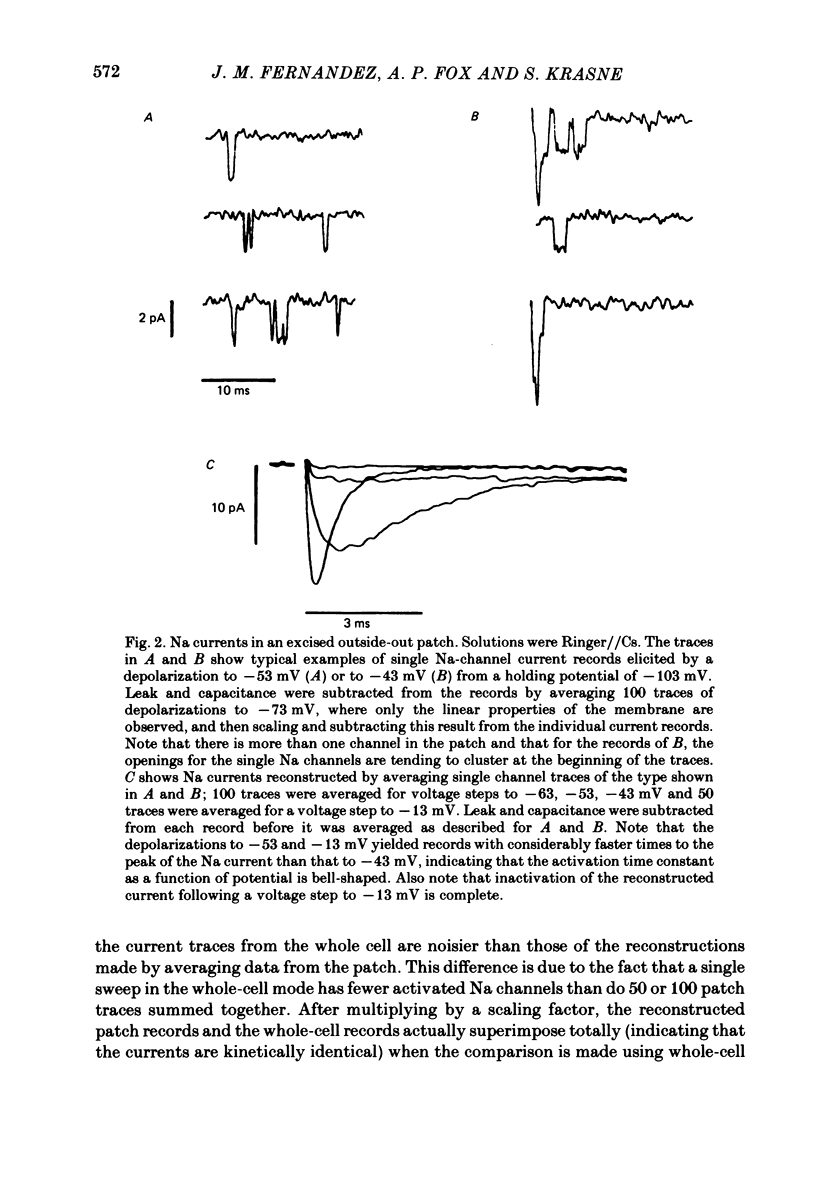
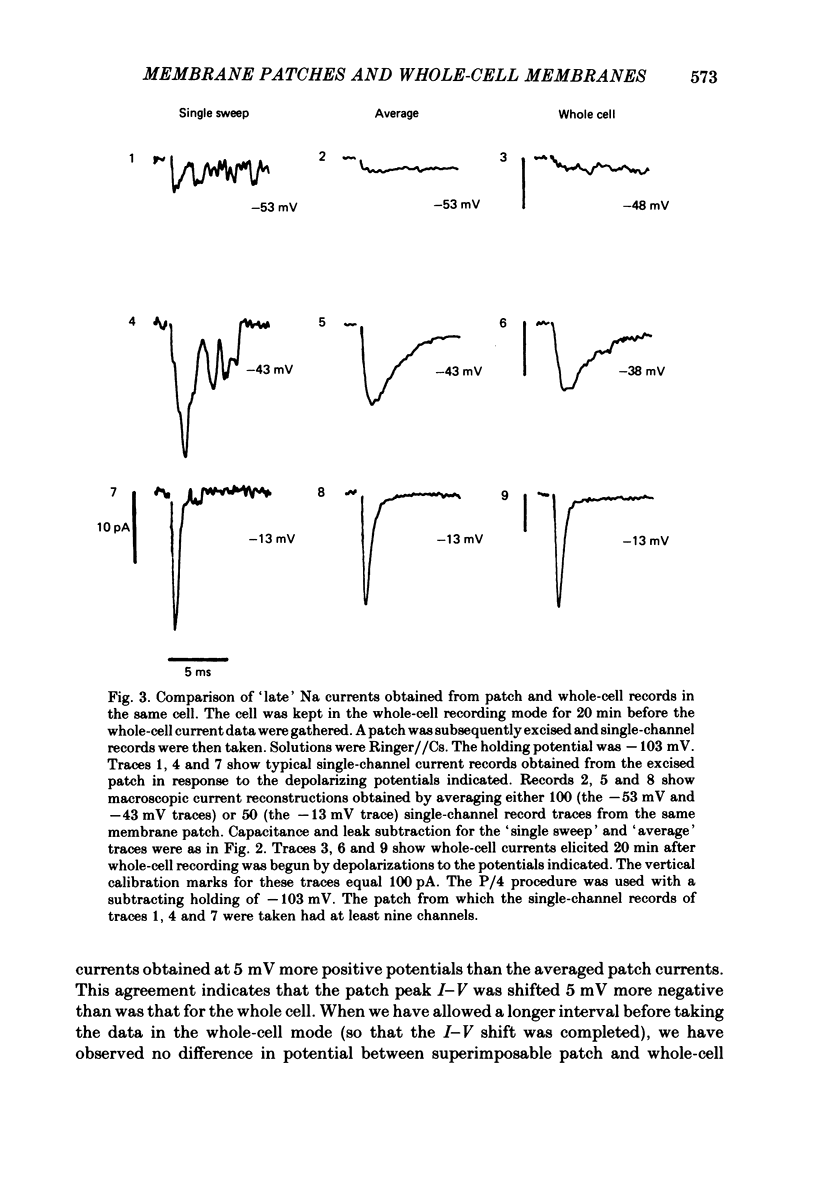
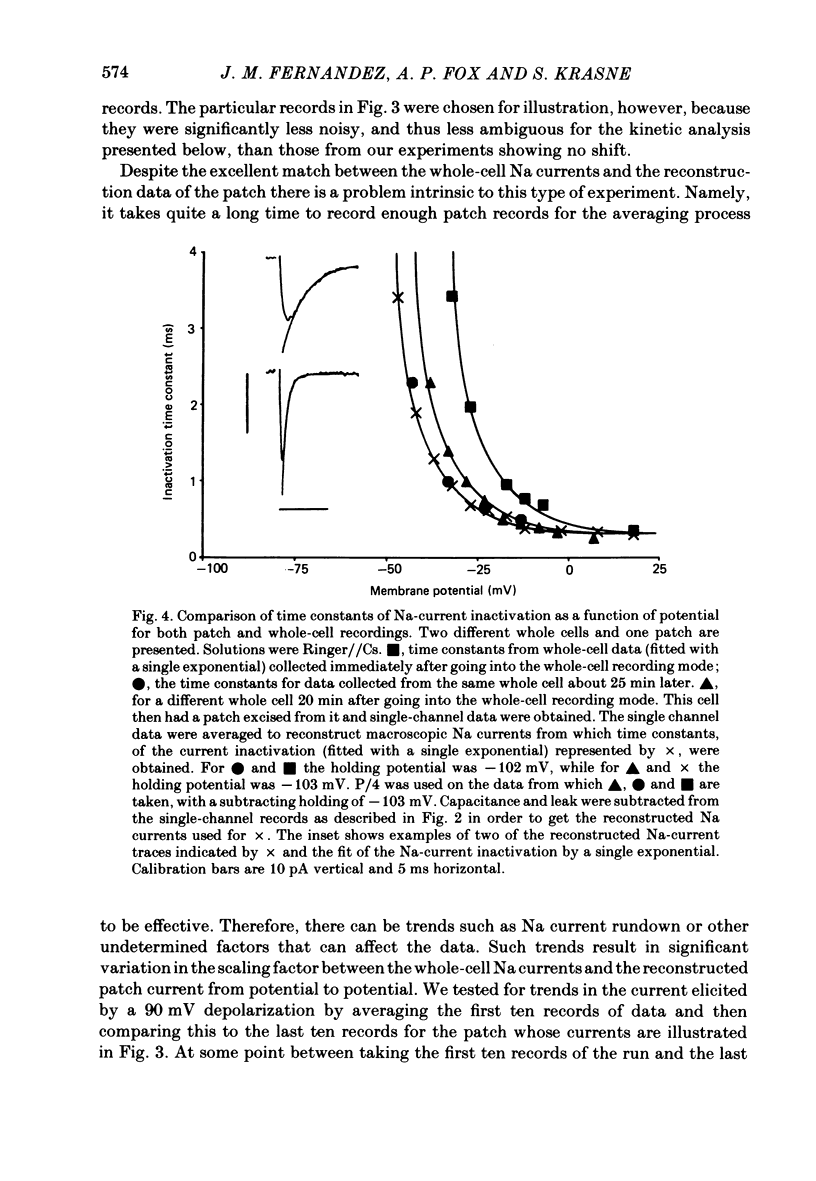
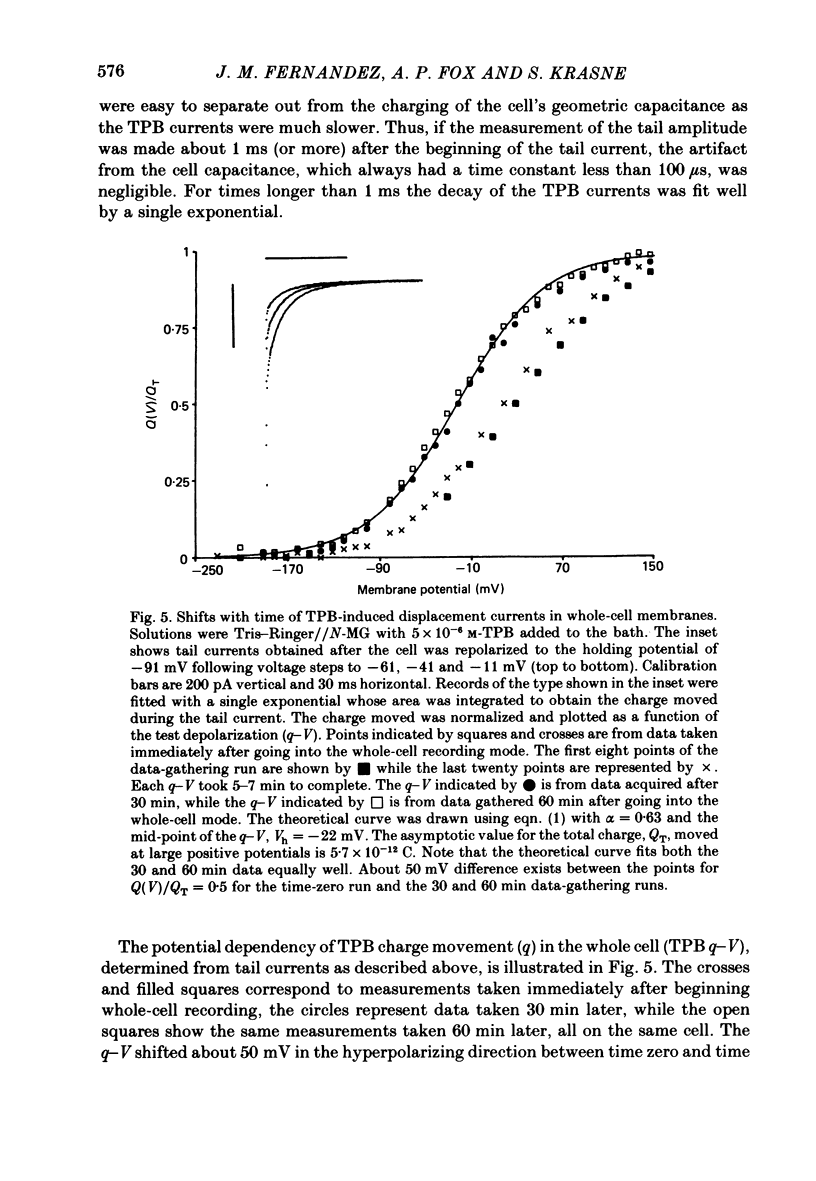
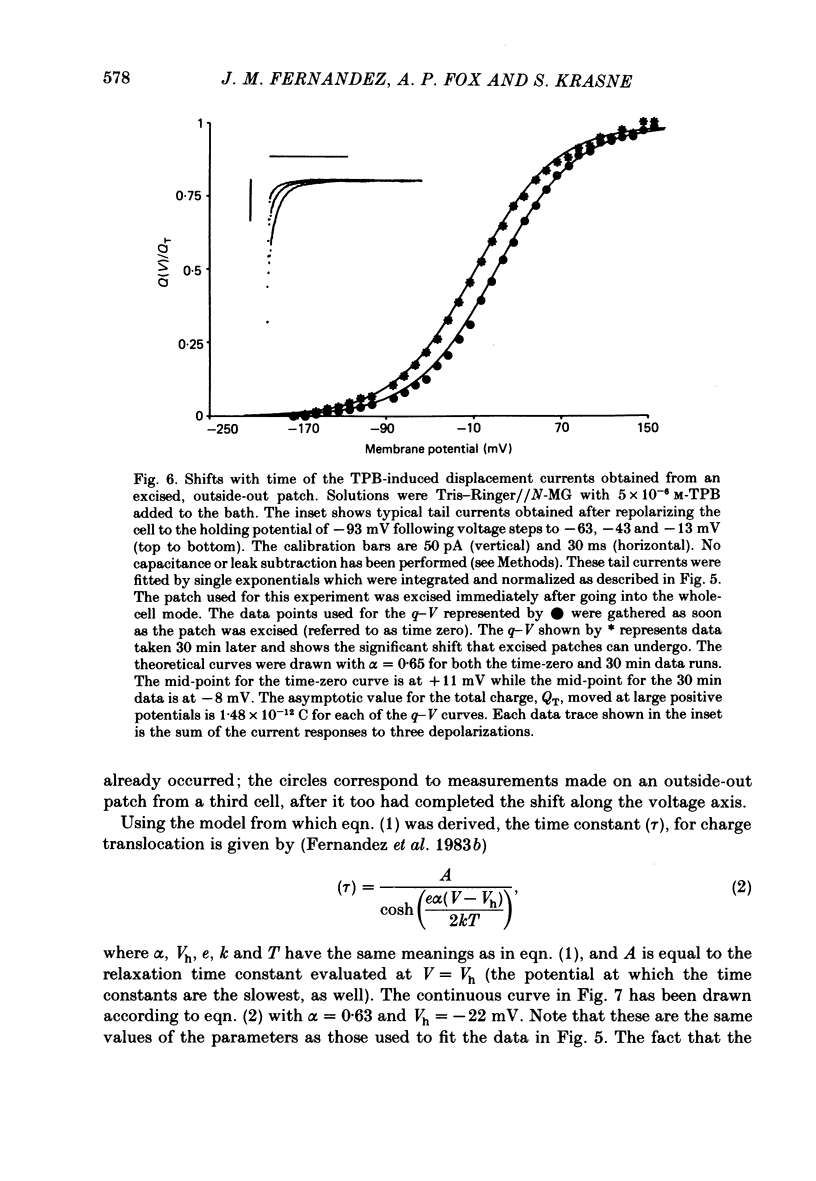
A comparison has been made between the electrical properties of excised 'outside-out' patches and whole-cell membranes of GH3 cells using the patch-pipette technique. Despite a complicated surface morphology, which includes numerous microvilli, ruffles and blebs, high-resistance seals (typically greater than 10(11) omega) were consistently formed between patch pipettes and GH3 cell membranes. When the internal solution contained 120 mM-CsF, outward currents through K channels were blocked and large Na channel currents were consistently observed in the whole-cell recording mode. Using the same solutions, single Na channel currents were readily observed in outside-out patches. Averaging patch currents yielded macroscopic currents showing the same voltage-dependent kinetics as those observed for the whole-cell membrane. The current vs. voltage and inactivation time constant vs. voltage relationships for the Na channel shifted towards more negative potentials (25 mV or more) within approximately 30 min after going into the whole-cell recording mode. These same relationships could be measured for outside-out patches and their positions along the voltage axis coincided with the asymptotic values measured in the whole-cell mode. When the internal solution contained 120 mM-N-methylglucamine fluoride and the external solution contained 120 mM-Tris chloride, no ionic channel currents could be observed either for whole-cell or outside-out patch membranes. Under these conditions, displacement currents induced by tetraphenyl borate (TPB) were recorded in both types of membranes. The total charge moved showed a sigmoidal dependence upon the applied voltage for both whole-cell and outside-out patch membranes. The charge vs. voltage relationship showed a shift along the voltage axis similar to that observed for Na channels except that the magnitude of the shift was larger. A shift in this relationship was also observed for excised patches but the observable magnitude of the shift was smaller than that in the whole-cell recording mode. The asymptotic values of the charge vs. voltage relationship were similar for whole-cell and outside-out patches, as were the asymptotic values for the translocation time constants. It is concluded that there are no fundamental differences in the properties of ionic channel and displacement currents between whole-cell membranes and excised membrane patches.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






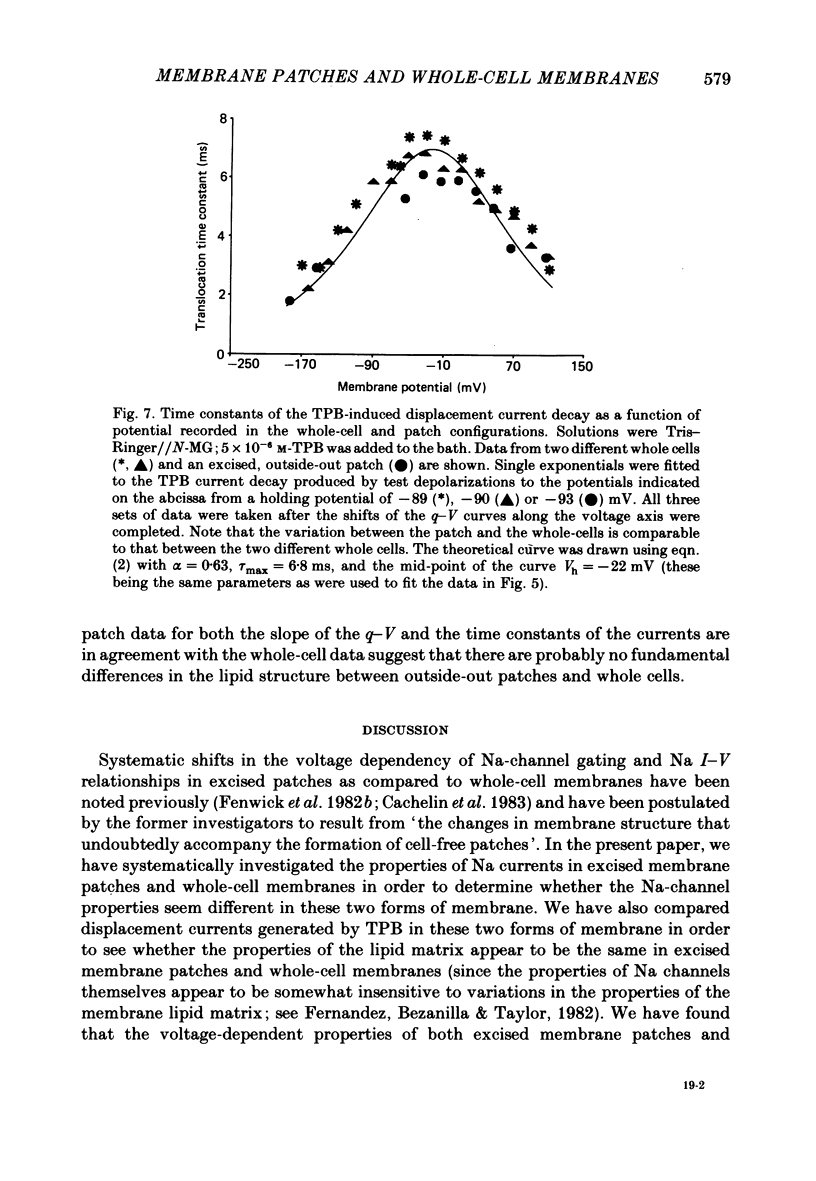






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Conti F. Structure of the squid axon membrane as derived from charge-pulse relaxation studies in the presence of absorbed lipophilic ions. J Membr Biol. 1981 Apr 15;59(2):91–104. doi: 10.1007/BF01875707. [DOI] [PubMed] [Google Scholar]
- Benz R., Nonner W. Structure of the axolemma of frog myelinated nerve: relaxation experiments with a lipophilic probe ion. J Membr Biol. 1981 Apr 15;59(2):127–134. doi: 10.1007/BF01875710. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández J. M., Bezanilla F., Taylor R. E. Effect of chloroform on charge movement in the nerve membrane. Nature. 1982 May 13;297(5862):150–152. doi: 10.1038/297150a0. [DOI] [PubMed] [Google Scholar]
- Fernández J. M., Taylor R. E., Bezanilla F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J Gen Physiol. 1983 Sep;82(3):331–346. doi: 10.1085/jgp.82.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J. Single channel currents from excised patches of muscle membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6930–6934. doi: 10.1073/pnas.77.11.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Effect of internal fluoride and phosphate on membrane currents during intracellular dialysis of nerve cells. Nature. 1975 Oct 23;257(5528):691–693. doi: 10.1038/257691a0. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Na and Ca channels in a transformed line of anterior pituitary cells. J Gen Physiol. 1984 Mar;83(3):371–394. doi: 10.1085/jgp.83.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Miyazaki S. I. Electrical excitability in the rat clonal pituitary cell and its relation to hormone secretion. Jpn J Physiol. 1979;29(4):411–426. doi: 10.2170/jjphysiol.29.411. [DOI] [PubMed] [Google Scholar]
- Reyes J., Latorre R. Effect of the anesthetics benzyl alcohol and chloroform on bilayers made from monolayers. Biophys J. 1979 Nov;28(2):259–279. doi: 10.1016/S0006-3495(79)85175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]